Recent sScientific evidence has shown an increased risk of fractures in patients with obesity, especially in those with a higher visceral adipose tissue content. This contradicts the old paradigm that obese patients were more protected than those with normal weight. Specifically, in older subjects in whom there is a redistribution of fat from subcutaneous adipose tissue to visceral adipose tissue and an infiltration of other tissues such as muscle with the consequent sarcopenia, obesity can accentuate the changes characteristic of this age group that predisposes to a greater risk of falls and fractures. Other factors that determine a greater risk in older subjects with obesity are chronic proinflammatory status, altered adipokine secretion, vitamin D deficiency, insulin resistance and reduced mobility.
- obesity
- fracture
- body composition
- inflammation
- healthy aging
- osteoporosis
1. Introduction
2. Pathophysiology
Given the results of epidemiological studies, various pathophysiological mechanisms have been investigated and described by which there could be a beneficial and/or detrimental relationship between obesity and bone fragility (Figure 1).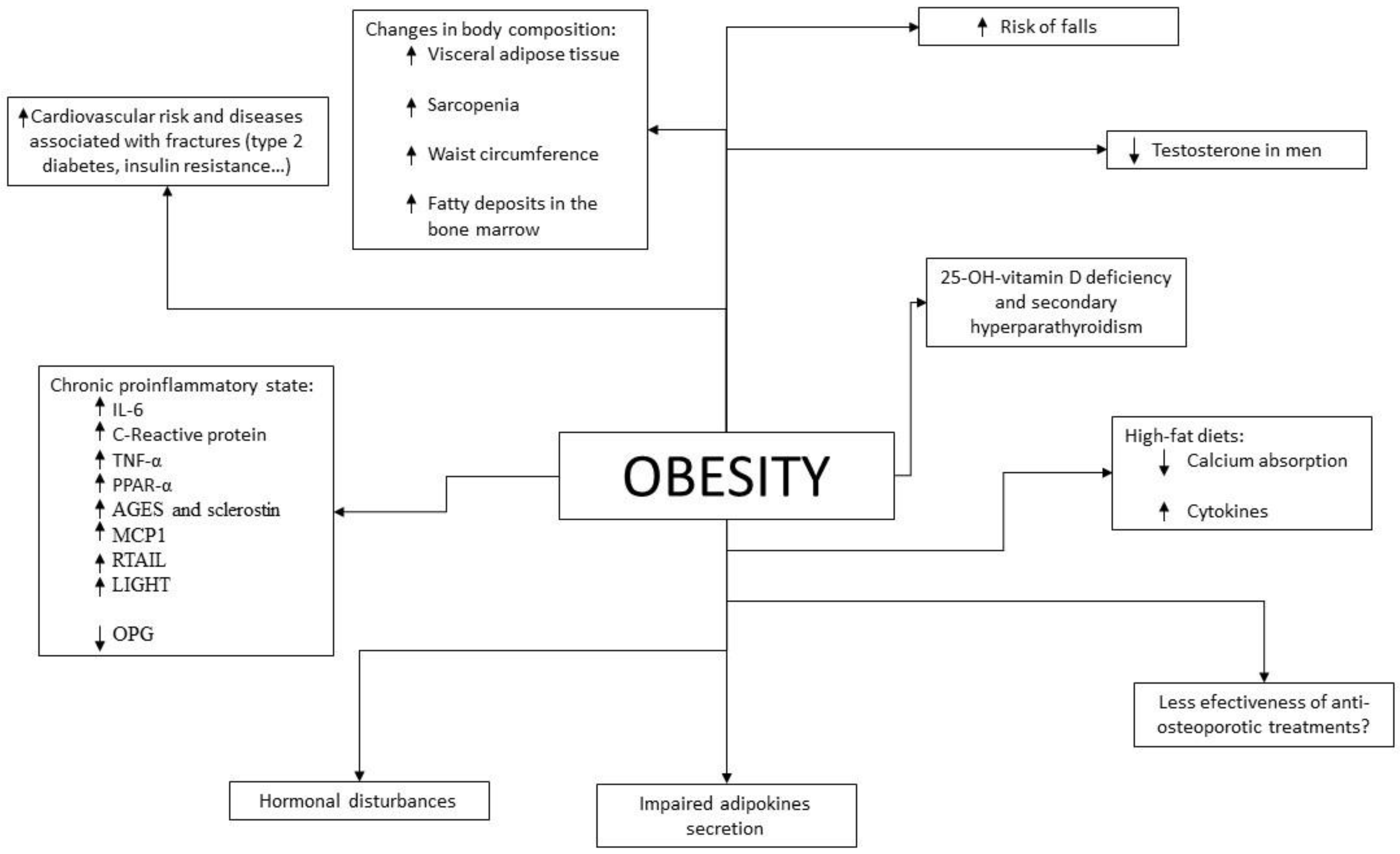
3. Changes in Body Composition during Aging
Aging produces various changes in body composition independently of changes in weight. In terms of muscle mass, between the ages of 24 and 50 years, 10% of muscle mass is lost, to which is added a 30% loss between the ages of 50 and 80 years, with a 1% annual decrease in the fifth decade of life [133][95]. This can lead to a state of sarcopenia, which is a state of decreased muscle mass and strength associated with functional limitations that may increase the risk of falls [134][96]. The prevalence of sarcopenia is estimated to be 5–13% in patients aged 60–70 years, increasing to 50% in patients aged 80 years or older and being more prevalent in patients with metabolic and chronic diseases [135][97]. One of the main characteristics of sarcopenia in the older subjects is fatty infiltration of muscle [136[98][99],137], which has been associated with an increased risk of fractures [138][100]. Obesity, due to the related chronic proinflammatory state, may contribute to a greater development of sarcopenia than that produced by aging itself [139][101]. The presence of obesity in patients with sarcopenia is referred to as sarcopenic obesity, which has been associated with an increased risk of morbidity and mortality [140][102]. Given the aging population and the increasing prevalence of obesity, this combination is becoming increasingly prevalent leading to a public health problem, especially given the resulting increase in cardiovascular risk [141][103]. It is estimated that the most important cause of fatty deposits in skeletal muscle is due to energy intake exceeding energy expenditure, resulting in energy storage in the form of adipose tissue. In people with obesity this is increased, since they have enlarged adipocytes in the subcutaneous tissue and an overload of lipid deposits that cause this excess fat to accumulate in other tissues such as muscle, following the “overflow hypothesis” [142][104]. In addition to this lipid overload, adipocytes in people with obesity have a lower capacity for lipid accumulation than adipocytes in people without obesity. This fact is due to the proinflammatory state of obesity, since the increased levels of IL-6 and TNF-α reduce the expression of PPAR-γ-2 and C/EBPα, which play an important role in the correct differentiation of preadipocytes into adipocytes [143][105]. On the other hand, the distribution of fat tissue itself also changes with aging, increasing visceral adipose tissue and decreasing subcutaneous adipose tissue, which goes on to infiltrate other organs such as muscle [144][106]. In particular, fatty infiltration of the bone marrow is relevant, which has been related to lower bone quality [145][107]. As we age, the capacity of preadipocytes to replicate, differentiate and resist apoptosis decreases due to the increase in inflammation parameters. This phenomenon is known as inflammaging [146][108]. As is mentioned, this redistribution is accentuated in patients with obesity also due to alteration of the regulatory mechanisms of inflammation. This change, especially due to the increase in visceral fat, increases cardiovascular risk in older subjects, with greater relevance in patients who are also obese. As previously described, the increase in visceral adipose tissue has also been associated with a decrease in BMD and an increased risk of fractures. Finally, it has been shown that older subjects may be particularly susceptible to the deleterious effects of obesity, since the correlation between BMI and frailty is U-shaped in these subjects, presenting the obese older subjects less aerobic capacity, less muscle strength, less physical performance and worse functionality [147][109]. In summary, aging produces changes in body composition (redistribution of adipose tissue with a decrease in subcutaneous fat and an increase in visceral, intramuscular—with the consequent sarcopenia—and bone marrow deposits) that are associated with greater bone fragility and an increased risk of falls and fractures. This redistribution is similar to that produced in obesity and therefore its deleterious effects are increased in older subjects and obese patients.4. Difficulties in the Diagnosis of Osteoporosis and Prediction of Fracture Risk in Patients with Obesity
As previously discussed, obese patients show higher BMD compared to patients without obesity on DXA. However, higher BMI and greater soft tissue thickness could alter this measurement [148][110]. In addition, it seems that BMD assessment by DXA may provide inappropriate values if not interpreted in relation to weight. As for other tests less widely used in daily clinical practice, such as high-resolution peripheral quantitative computed tomography (HRpQCT), a greater BMD has also been shown in patients with obesity, as well as greater cortical and trabecular BMD and a greater number of trabeculae in the distal radius and distal tibia, where they also present greater bone strength [149,150][111][112]. However, the bone size in the tibia and radius measured by this technique is not increased with respect to patients with normal weight, unlike the hip area [149,151][111][113]. This is in contradiction with the theory that mechanical overload in patients with obesity would contribute to increased bone formation. This technique also allows the calculation of the amount of adipose tissue in the bone marrow, which is usually increased in patients with obesity and in the older subjects and which has been related to bone microstructural deterioration and the presence of non-vertebral fractures [152][114]. Like DXA, the accuracy of this test is also influenced by the thickness of the soft tissue [153][115]. In patients with type 2 diabetes, a cortical strength deficit has been observed by HRpQCT, due to reduced cortical thickness and volume with increased cortical porosity in patients with microvascular complications [154][116]. This has also been found to be increased in patients with type 2 diabetes with previous fractures, so it seems that these changes would contribute to an increased risk of fractures in these patients [155][117]. As for bone remodeling markers, these are found to be decreased in patients with obesity when compared to patients with normal weight, this difference being greater in bone resorption markers than in bone formation markers [156][118]. This reduction has also been demonstrated in patients with type 2 diabetes, independently of glucose levels [157][119], which is in agreement with the results of histomorphometric studies in which signs compatible with low bone remodeling are observed [158][120]. Regarding fracture risk, tools such as FRAX can underestimate it in these patients. As is known, given the description of the increase in fractures in relation to BMIs below normal, this is a parameter that is considered in this algorithm. However, given the results of older epidemiological studies previously discussed, obesity is not included as a risk factor for fractures in this tool. There are studies that have evaluated the sensitivity of FRAX in this group of patients. In 2013, Premaor et al. [159][121] compared obese postmenopausal women with non-obese women, observing that the probability calculated by FRAX for fracture at 10 years was significantly lower in the first group (7.1% vs. 10.9% in hip fracture and 18.2% vs. 23.3% in major osteoporotic fracture respectively), even if BMI was not included in the calculation (5.8% vs. 11.4% in hip fracture and 17.6% vs. 23.6% in major osteoporotic fracture). Despite this, when calculating the ROC curve, the area under the curve was similar in both groups with and without the inclusion of BMI in the calculation. It therefore suggests that the cut-off values at which to intervene may be too high for patients with obesity and lower reference values should be considered for initiating treatment. Moreover, the percentages of predicted and subsequently observed fractures were similar between groups. Another study conducted in 2014 [160][122] also showed that the ability of FRAX to predict fractures did not vary with body composition. However, the FRAX tool has two important limitations in patients with obesity: the first is that it does not predict fractures that are more frequent in this group of patients, such as ankle fractures; the second is that in patients with type 2 diabetes, increased waist circumference and/or insulin resistance it has been shown to underestimate the risk of fracture [161][123]. Considering the current prevalence of obesity in older subjects, more studies are needed in the coming years to clarify this issue because of its implications. It should be noted that, as mentioned above, patients with obesity who undergo an osteoporosis study should also be asked to have their HbA1c level measured, since diabetes influences the interpretation of the tests.5. Prevention of Osteoporosis and Fractures in Older Subjects with Obesity
For the prevention of osteoporosis and fractures in patients with obesity, special emphasis should be placed on lifestyle measures. As in the general population, smoking and alcohol intake cessation should be advised, as well as physical exercise and a healthy diet. Weight loss has been associated with a 1–4% loss of bone mass in the hip and trabecular bones [162[124][125][126][127],163,164,165], especially in older subjects [166,167][128][129]. When it occurs involuntarily, it has been associated with an increase in hip and upper limb fractures [168][130], but this may be due to the loss of muscle mass that occurs when weight is lost involuntarily rather than the weight reduction itself. Studies that have evaluated intentional weight loss have shown increases in lower leg risk but a decrease in hip, pelvic and spine fractures [168][130]. That distribution of fractures is similar to that occurring in patients with obesity, who are the most likely to undertake an intentional weight loss program, so these results could be biased. Furthermore, recent studies have shown that when weight loss is moderate, BMD is not reduced and bone geometry is not altered [169][131]. Compared to this moderate weight loss, intense caloric restriction in a randomized clinical trial resulted in a greater loss of BMD in the hip in postmenopausal women, but not in the lumbar spine [170][132]. In this same group of patients, a study showed that when BMD is lost after weight loss, it does not recover if the lost weight is regained [171][133]. Given the relevance of sarcopenia in obese older patients with respect to the risk of osteoporosis and fractures, multiple studies have evaluated the role of physical exercise in these weight loss programs. In older obese patients undergoing a weight loss program, physical exercise has been shown to reduce frailty and decrease BMD and sarcopenia [147,172,173][109][134][135] with both resistance exercise programs [173][135] and aerobic combined with resistance exercise programs [172][134]. On the other hand, dairy intake during weight loss has been associated with higher osteocalcin levels and increased BMD in the lumbar spine when compared to low dairy intake [174][136]. In summary, in older patients with obesity, moderate weight loss should be advised in a program that includes adequate dairy intake and resistance exercise. Regarding diet, it has been described, in experimental models, that hypercaloric and obesogenic diets are related to an increased risk of fracture by direct and indirect mechanisms [175,176][137][138]. High-fat diets are a risk factor for osteoporosis. In mice subjected to this type of diet, T lymphocytes isolated from the spleen and bone marrow showed increased expression of RANKL, and these mice had decreased BMD [177][139], as well as increased levels of cytokines such as IL-6 and TNF-α. It has also been shown in animal models that this type of diet affects bone remodeling, triggering a loss of trabecular bone mass and also reduces calcium absorption [178][140]. On the other hand, a high-fat and high-sucrose diet has been shown to affect the cortical bone in mice and rats, especially when maintained over the long term [179,180,181][141][142][143]. In humans, data regarding the effect of a high-fat diet on the risk of osteoporosis and fracture are scarce and contradictory [182][144]. However, some prospective and cross-sectional studies have shown a protective effect with protein intake [182][144]. It is also worth noting the importance in these patients of an adequate intake of calcium and vitamin D, since as indicated above, high-fat diets tend to decrease calcium absorption and these patients have a high prevalence of vitamin D deficiency. As in the general population, it is recommended to obtain an optimal calcium and vitamin D intake through diet and not with supplementation if possible, especially with regard to calcium supplements that could increase arteriosclerosis [183][145]. As for vitamin D, given its accumulation in adipose tissue, higher doses are usually required than in the general population. Current measures to reduce osteoporosis and fracture risk in obesity are shown in Figure 2.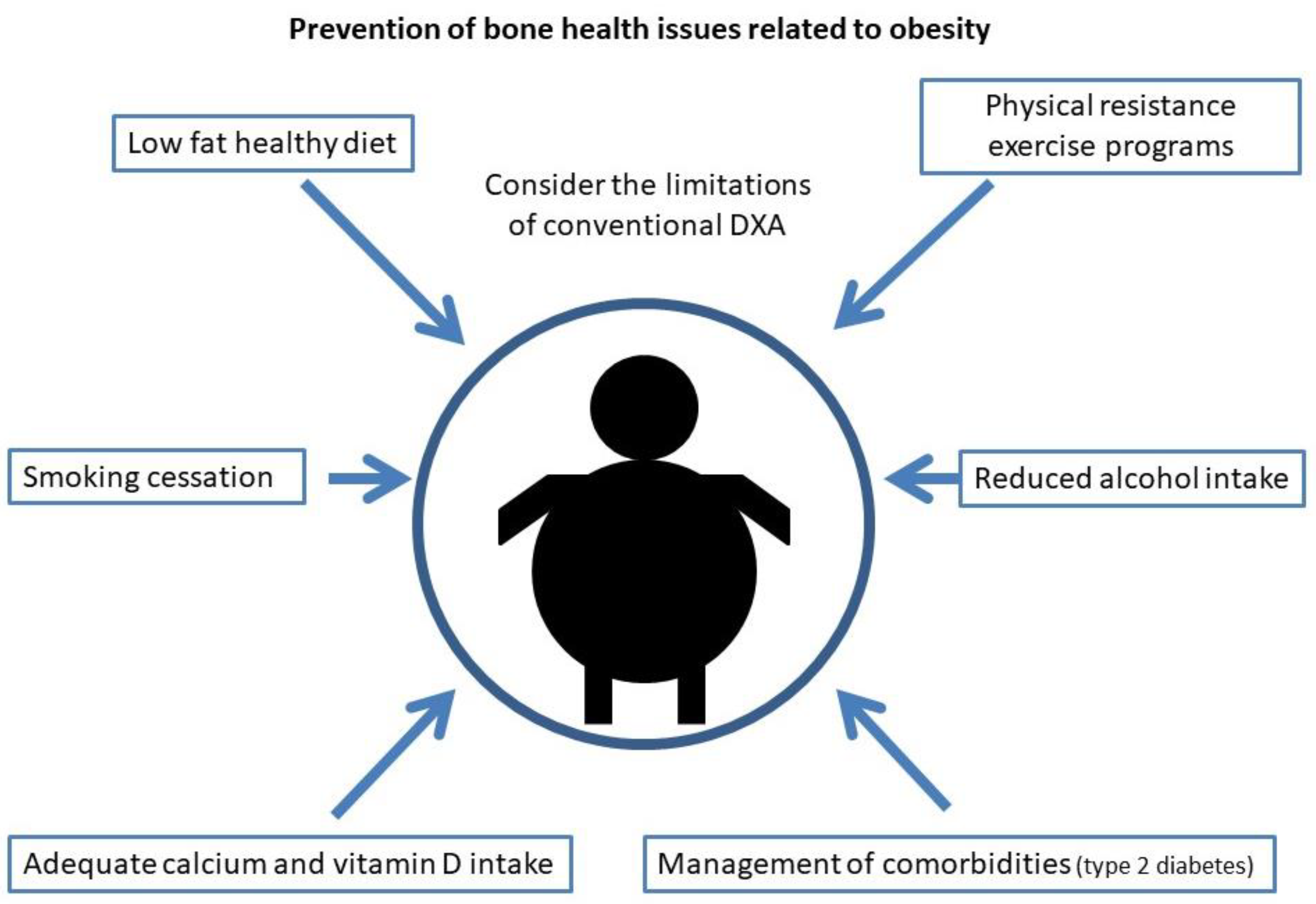
References
- Greco, E.A.; Lenzi, A.; Migliaccio, S. The obesity of bone. Ther. Adv. Endocrinol. Metab. 2015, 6, 273–286.
- National Institutes of Health. Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795.
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381.
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General; US Department of Health and Human Services: Rockville, MD, USA, 2004.
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 March 2022).
- Cawley, J.; Meyerhoefer, C. The medical care costs of obesity: An instrumental variables approach. J. Health Econ. 2012, 31, 219–230.
- Dytfeld, J.; Michalak, M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: Meta-analysis of observational studies. Aging Clin. Exp. Res. 2017, 29, 301–309.
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos. Int. 2007, 18, 427–444.
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505.
- Jia, P.; Bao, L.; Chen, H.; Yuan, J.; Liu, W.; Feng, F.; Li, J.; Tang, H. Risk of low-energy fracture in type 2 diabetes patients: A meta-analysis of observational studies. Osteoporos. Int. 2017, 28, 3113–3121.
- Bai, J.; Gao, Q.; Wang, C.; Dai, J. Diabetes mellitus and risk of low-energy fracture: A meta-analysis. Aging Clin. Exp. Res. 2020, 32, 2173–2186.
- Vilaca, T.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone 2020, 137, 115457.
- Walsh, J.S.; Vilaca, T. Obesity, Type 2 Diabetes and Bone in Adults. Calcif. Tissue Res. 2017, 100, 528–535.
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Res. 1995, 57, 344–358.
- Premaor, M.O.; Pilbrow, L.; Tonkin, C.; Parker, R.A.; Compston, J. Obesity and fractures in postmenopausal women. J. Bone Miner. Res. 2010, 25, 292–297.
- Compston, J. Obesity and fractures in postmenopausal women. Curr. Opin. Rheumatol. 2015, 27, 414–419.
- Compston, J. Obesity and bone. Curr. Osteoporos. Rep. 2013, 11, 30–35.
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Díez-Pérez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050.
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21.
- Emmanuelle, N.-E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568.
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211.
- Orwoll, E.; Lambert, L.C.; Marshall, L.M.; Blank, J.; Barrett-Connor, E.; Cauley, J.; Ensrud, K.; Cummings, S.R. Osteoporotic Fractures in Men Study Group Endogenous testosterone levels, physical performance, and fall risk in older men. Arch. Intern. Med. 2006, 166, 2124–2131.
- Paller, C.J.; Shiels, M.S.; Rohrmann, S.; Basaria, S.; Rifai, N.; Nelson, W.; Platz, E.A.; Dobs, A. Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clin. Endocrinol. (Oxf.) 2009, 70, 26–34.
- Devine, A.; Dick, I.M.; Dhaliwal, S.S.; Naheed, R.; Beilby, J.; Prince, R.L. Prediction of incident osteoporotic fractures in elderly women using the free estradiol index. Osteoporos. Int. 2005, 16, 216–221.
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.a.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349.
- Sukumar, D.; Schlussel, Y.; Riedt, C.S.; Gordon, C.; Stahl, T.; Shapses, S.A. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos. Int. 2011, 22, 635–645.
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30.
- Gautier, A.; Bonnet, F.; Dubois, S.; Massart, C.; Grosheny, C.; Bachelot, A.; Aubé, C.; Balkau, B.; Ducluzeau, P.-H. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin. Endocrinol. (Oxf.) 2013, 78, 373–378.
- Baldini, V.; Mastropasqua, M.; Francucci, C.M.; D’Erasmo, E. Cardiovascular disease and osteoporosis. J. Endocrinol. Investig. 2005, 28, 69–72.
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Harrington, L.M.; Breggia, A.; Rosen, C.J.; Miller, K.K. Determinants of bone mineral density in obese premenopausal women. Bone 2011, 48, 748–754.
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300.
- Isaia, G.C.; D’Amelio, P.; Di Bella, S.; Tamone, C. Is leptin the link between fat and bone mass? J. Endocrinol. Investig. 2005, 28, 61–65.
- Shinoda, Y.; Yamaguchi, M.; Ogata, N.; Akune, T.; Kubota, N.; Yamauchi, T.; Terauchi, Y.; Kadowaki, T.; Takeuchi, Y.; Fukumoto, S.; et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J. Cell. Biochem. 2006, 99, 196–208.
- Luo, X.-H.; Guo, L.-J.; Yuan, L.-Q.; Xie, H.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp. Cell Res. 2005, 309, 99–109.
- Luo, X.-H.; Guo, L.-J.; Xie, H.; Yuan, L.-Q.; Wu, X.-P.; Zhou, H.-D.; Liao, E.-Y. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J. Bone Miner. Res. 2006, 21, 1648–1656.
- Lenchik, L.; Register, T.C.; Hsu, F.C.; Lohman, K.; Nicklas, B.J.; Freedman, B.I.; Langefeld, C.D.; Carr, J.J.; Bowden, D.W. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 2003, 33, 646–651.
- Jürimäe, J.; Rembel, K.; Jürimäe, T.; Rehand, M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm. Metab. Res. 2005, 37, 297–302.
- Jürimäe, J.; Jürimäe, T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos. Int. 2007, 18, 1253–1259.
- Zoico, E.; Zamboni, M.; Di Francesco, V.; Mazzali, G.; Fantin, F.; De Pergola, G.; Zivelonghi, A.; Adami, S.; Bosello, O. Relation between adiponectin and bone mineral density in elderly post-menopausal women: Role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J. Endocrinol. Investig. 2008, 31, 297–302.
- Richards, J.B.; Valdes, A.M.; Burling, K.; Perks, U.C.; Spector, T.D. Serum adiponectin and bone mineral density in women. J. Clin. Endocrinol. Metab. 2007, 92, 1517–1523.
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935.
- Thomas, T. The complex effects of leptin on bone metabolism through multiple pathways. Curr. Opin. Pharmacol. 2004, 4, 295–300.
- Lamghari, M.; Tavares, L.; Camboa, N.; Barbosa, M.A. Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J. Cell. Biochem. 2006, 98, 1123–1129.
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207.
- Zoico, E.; Zamboni, M.; Adami, S.; Vettor, R.; Mazzali, G.; Tosoni, P.; Bissoli, L.; Bosello, O. Relationship between leptin levels and bone mineral density in the elderly. Clin. Endocrinol. (Oxf.) 2003, 59, 97–103.
- Holecki, M.; Wiecek, A. Relationship between body fat mass and bone metabolism. Pol. Arch. Intern. Med. 2010, 120, 361–367.
- Couce, M.E.; Green, D.; Brunetto, A.; Achim, C.; Lloyd, R.V.; Burguera, B. Limited brain access for leptin in obesity. Pituitary 2001, 4, 101–110.
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334.
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415.
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43.
- Bennett, J.H.; Joyner, C.J.; Triffitt, J.T.; Owen, M.E. Adipocytic cells cultured from marrow have osteogenic potential. J. Cell Sci. 1991, 99, 131–139.
- Pei, L.; Tontonoz, P. Fat’s loss is bone’s gain. J. Clin. Investig. 2004, 113, 805–806.
- Kirkland, J.L.; Tchkonia, T.; Pirtskhalava, T.; Han, J.; Karagiannides, I. Adipogenesis and aging: Does aging make fat go MAD? Exp. Gerontol. 2002, 37, 757–767.
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055.
- Pfeilschifter, J.; Köditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119.
- Moelants, E.A.V.; Mortier, A.; Van Damme, J.; Proost, P. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013, 91, 393–401.
- Xu, F.; Du, Y.; Hang, S.; Chen, A.; Guo, F.; Xu, T. Adipocytes regulate the bone marrow microenvironment in a mouse model of obesity. Mol. Med. Rep. 2013, 8, 823–828.
- Zheng, L.-W.; Wang, W.-C.; Mao, X.-Z.; Luo, Y.-H.; Tong, Z.-Y.; Li, D. TNF-α regulates the early development of avascular necrosis of the femoral head by mediating osteoblast autophagy and apoptosis via the p38 MAPK/NF-κB signaling pathway. Cell Biol. Int. 2020, 44, 1881–1889.
- Kim, J.A.; Roh, E.; Hong, S.-H.; Lee, Y.-B.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, N.H.; Kim, S.G.; Baik, S.H.; et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone 2019, 128, 115053.
- Van Damme, J.; Proost, P.; Put, W.; Arens, S.; Lenaerts, J.P.; Conings, R.; Opdenakker, G.; Heremans, H.; Billiau, A. Induction of monocyte chemotactic proteins MCP-1 and MCP-2 in human fibroblasts and leukocytes by cytokines and cytokine inducers. Chemical synthesis of MCP-2 and development of a specific RIA. J. Immunol. 1994, 152, 5495–5502.
- Torzewski, J.; Oldroyd, R.; Lachmann, P.; Fitzsimmons, C.; Proudfoot, D.; Bowyer, D. Complement-induced release of monocyte chemotactic protein-1 from human smooth muscle cells. A possible initiating event in atherosclerotic lesion formation. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 673–677.
- Brown, Z.; Strieter, R.M.; Neild, G.H.; Thompson, R.C.; Kunkel, S.L.; Westwick, J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992, 42, 95–101.
- Villiger, P.M.; Terkeltaub, R.; Lotz, M. Monocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage. Induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acid. J. Clin. Investig. 1992, 90, 488–496.
- Barna, B.P.; Pettay, J.; Barnett, G.H.; Zhou, P.; Iwasaki, K.; Estes, M.L. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 1994, 50, 101–107.
- Matsushima, K.; Larsen, C.G.; DuBois, G.C.; Oppenheim, J.J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J. Exp. Med. 1989, 169, 1485–1490.
- Colotta, F.; Borré, A.; Wang, J.M.; Tattanelli, M.; Maddalena, F.; Polentarutti, N.; Peri, G.; Mantovani, A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J. Immunol. 1992, 148, 760–765.
- Seitz, M.; Loetscher, P.; Dewald, B.; Towbin, H.; Gallati, H.; Baggiolini, M. Interleukin-10 differentially regulates cytokine inhibitor and chemokine release from blood mononuclear cells and fibroblasts. Eur. J. Immunol. 1995, 25, 1129–1132.
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302.
- Van Damme, J.; Proost, P.; Lenaerts, J.P.; Opdenakker, G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J. Exp. Med. 1992, 176, 59–65.
- Cochran, B.H.; Reffel, A.C.; Stiles, C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell 1983, 33, 939–947.
- Kim, M.S.; Day, C.J.; Selinger, C.I.; Magno, C.L.; Stephens, S.R.J.; Morrison, N.A. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J. Biol. Chem. 2006, 281, 1274–1285.
- Brunetti, G.; Oranger, A.; Carbone, C.; Mori, G.; Sardone, F.R.; Mori, C.; Celi, M.; Faienza, M.F.; Tarantino, U.; Zallone, A.; et al. Osteoblasts display different responsiveness to TRAIL-induced apoptosis during their differentiation process. Cell Biophys. Biophys 2013, 67, 1127–1136.
- Brunetti, G.; Oranger, A.; Mori, G.; Sardone, F.; Pignataro, P.; Coricciati, M.; Napoli, N.; Rizzi, R.; Liso, V.; Grassi, F.R.; et al. TRAIL effect on osteoclast formation in physiological and pathological conditions. Front. Biosci. (Elite Ed.) 2011, 3, 1154–1161.
- Zoller, V.; Funcke, J.-B.; Roos, J.; Dahlhaus, M.; Abd El Hay, M.; Holzmann, K.; Marienfeld, R.; Kietzmann, T.; Debatin, K.-M.; Wabitsch, M.; et al. Trail (TNF-related apoptosis-inducing ligand) induces an inflammatory response in human adipocytes. Sci. Rep. 2017, 7, 5691.
- Funcke, J.-B.; Zoller, V.; El Hay, M.A.; Debatin, K.-M.; Wabitsch, M.; Fischer-Posovszky, P. TNF-related apoptosis-inducing ligand promotes human preadipocyte proliferation via ERK1/2 activation. FASEB J. 2015, 29, 3065–3075.
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocrine-Related Cancer 2009, 16, 1103–1123.
- Chang, Y.-H.; Lin, K.-D.; He, S.-R.; Hsieh, M.-C.; Hsiao, J.-Y.; Shin, S.-J. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism 2011, 60, 1064–1069.
- Ugur-Altun, B.; Altun, A.; Gerenli, M.; Tugrul, A. The relationship between insulin resistance assessed by HOMA-IR and serum osteoprotegerin levels in obesity. Diabetes Res. Clin. Pract. 2005, 68, 217–222.
- Holecki, M.; Zahorska-Markiewicz, B.; Janowska, J.; Nieszporek, T.; Wojaczyńska-Stanek, K.; Zak-Gołab, A.; Wiecek, A. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity (Silver Spring) 2007, 15, 1925–1929.
- Yilmaz, Y.; Yonal, O.; Kurt, R.; Oral, A.Y.; Eren, F.; Ozdogan, O.; Ari, F.; Celikel, C.A.; Korkmaz, S.; Ulukaya, E.; et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand. J. Clin. Lab. Investig. 2010, 70, 541–546.
- Gannagé-Yared, M.-H.; Yaghi, C.; Habre, B.; Khalife, S.; Noun, R.; Germanos-Haddad, M.; Trak-Smayra, V. Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: Results from both cross-sectional and interventional study. Eur. J. Endocrinol. 2008, 158, 353–359.
- Brunetti, G.; Rizzi, R.; Oranger, A.; Gigante, I.; Mori, G.; Taurino, G.; Mongelli, T.; Colaianni, G.; Di Benedetto, A.; Tamma, R.; et al. LIGHT/TNFSF14 increases osteoclastogenesis and decreases osteoblastogenesis in multiple myeloma-bone disease. Oncotarget 2014, 5, 12950–12967.
- Cafiero, C.; Gigante, M.; Brunetti, G.; Simone, S.; Chaoul, N.; Oranger, A.; Ranieri, E.; Colucci, S.; Pertosa, G.B.; Grano, M.; et al. Inflammation induces osteoclast differentiation from peripheral mononuclear cells in chronic kidney disease patients: Crosstalk between the immune and bone systems. Nephrol. Dial. Transplant. 2018, 33, 65–75.
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830.
- Blüher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250.
- Gonnelli, S.; Caffarelli, C.; Del Santo, K.; Cadirni, A.; Guerriero, C.; Lucani, B.; Franci, B.; Nuti, R. The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif. Tissue Res. 2008, 83, 55–60.
- Reid, I.R. Fat and bone. Arch. Biochem. Biophys. 2010, 503, 20–27.
- Fulzele, K.; Clemens, T.L. Novel functions for insulin in bone. Bone 2012, 50, 452–456.
- Clemens, T.L.; Karsenty, G. The osteoblast: An insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 2011, 26, 677–680.
- Shin, D.; Kim, S.; Kim, K.H.; Lee, K.; Park, S.M. Association between insulin resistance and bone mass in men. J. Clin. Endocrino.l Metab. 2014, 99, 988–995.
- Choi, Y.J.; Kim, D.J.; Lee, Y.; Chung, Y.-S. Insulin is inversely associated with bone mass, especially in the insulin-resistant population: The Korea and US National Health and Nutrition Examination Surveys. J. Clin. Endocrinol. Metab. 2014, 99, 1433–1441.
- Fukushima, N.; Hanada, R.; Teranishi, H.; Fukue, Y.; Tachibana, T.; Ishikawa, H.; Takeda, S.; Takeuchi, Y.; Fukumoto, S.; Kangawa, K.; et al. Ghrelin directly regulates bone f.formation. J. Bone Miner. Res. 2005, 20, 790–798.
- Napoli, N.; Pedone, C.; Pozzilli, P.; Lauretani, F.; Ferrucci, L.; Incalzi, R.A. Adiponectin and bone mass density: The InCHIANTI study. Bone 2010, 47, 1001–1005.
- Carrasco, F.; Basfi-Fer, K.; Rojas, P.; Valencia, A.; Csendes, A.; Codoceo, J.; Inostroza, J.; Ruz, M. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: Relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes. Surg. 2014, 24, 877–884.
- Deschenes, M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004, 34, 809–824.
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. 2005, 60, 324–333.
- Dodds, R.M.; Roberts, H.C.; Cooper, C.; Sayer, A.A. The Epidemiology of Sarcopenia. J. Clin. Densitom. 2015, 18, 461–466.
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618.
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585.
- Schafer, A.L.; Vittinghoff, E.; Lang, T.F.; Sellmeyer, D.E.; Harris, T.B.; Kanaya, A.M.; Strotmeyer, E.S.; Cawthon, P.M.; Cummings, S.R.; Tylavsky, F.A.; et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J. Clin. Endocrinol. Metab. 2010, 95, E368–E372.
- Kim, T.N.; Park, M.S.; Ryu, J.Y.; Choi, H.Y.; Hong, H.C.; Yoo, H.J.; Kang, H.J.; Song, W.; Park, S.W.; Baik, S.H.; et al. Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: The Korean Sarcopenic Obesity Study (KSOS). PLoS ONE 2014, 9, e115407.
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537.
- Atkins, J.L.; Wannamathee, S.G. Sarcopenic obesity in ageing: Cardiovascular outcomes and mortality. Br. J. Nutr. 2020, 124, 1102–1113.
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age--An overview. Exp. Gerontol. 2016, 76, 25–32.
- Gustafson, B.; Smith, U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J. Biol. Chem. 2006, 281, 9507–9516.
- Akazawa, N.; Kishi, M.; Hino, T.; Tsuji, R.; Tamura, K.; Hioka, A.; Moriyama, H. Intramuscular adipose tissue in the quadriceps is more strongly related to recovery of activities of daily living than muscle mass in older inpatients. J. Cachexia Sarcopenia Muscle 2021, 12, 891–899.
- Schellinger, D.; Lin, C.S.; Hatipoglu, H.G.; Fertikh, D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am. J. Neuroradiol. 2001, 22, 1620–1627.
- Cartwright, M.J.; Tchkonia, T.; Kirkland, J.L. Aging in adipocytes: Potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 2007, 42, 463–471.
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 2004, 12, 913–920.
- Knapp, K.M.; Welsman, J.R.; Hopkins, S.J.; Fogelman, I.; Blake, G.M. Obesity increases precision errors in dual-energy X-ray absorptiometry measurements. J. Clin. Densitom. 2012, 15, 315–319.
- Evans, A.L.; Paggiosi, M.A.; Eastell, R.; Walsh, J.S. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 2015, 30, 920–928.
- Sornay-Rendu, E.; Boutroy, S.; Vilayphiou, N.; Claustrat, B.; Chapurlat, R.D. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: The Os des Femmes de Lyon (OFELY) study. J. Bone Miner. Res. 2013, 28, 1679–1687.
- Shen, J.; Nielson, C.M.; Marshall, L.M.; Lee, D.C.; Keaveny, T.M.; Orwoll, E.S. Osteoporotic Fractures in Men MrOS Research Group The Association Between BMI and QCT-Derived Proximal Hip Structure and Strength in Older Men: A Cross-Sectional Study. J. Bone Miner. Res. 2015, 30, 1301–1308.
- Zebaze, R.; Osima, M.; Bui, M.; Lukic, M.; Wang, X.; Ghasem-Zadeh, A.; Eriksen, E.F.; Vais, A.; Shore-Lorenti, C.; Ebeling, P.R.; et al. Adding Marrow Adiposity and Cortical Porosity to Femoral Neck Areal Bone Mineral Density Improves the Discrimination of Women With Nonvertebral Fractures From Controls. J. Bone Miner. Res. 2019, 34, 1451–1460.
- Wu, P.-H.; Gupta, T.; Chang, H.; Petrenko, D.; Schafer, A.; Kazakia, G. Soft tissue variations influence HR-pQCT density measurements in a spatially dependent manner. Bone 2020, 138, 115505.
- Shanbhogue, V.V.; Hansen, S.; Frost, M.; Jørgensen, N.R.; Hermann, A.P.; Henriksen, J.E.; Brixen, K. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur. J. Endocrinol. 2016, 174, 115–124.
- Patsch, J.M.; Burghardt, A.J.; Yap, S.P.; Baum, T.; Schwartz, A.V.; Joseph, G.B.; Link, T.M. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J. Bone Miner. Res. 2013, 28, 313–324.
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone Miner. Res. 2000, 15, 1526–1536.
- Starup-Linde, J.; Eriksen, S.A.; Lykkeboe, S.; Handberg, A.; Vestergaard, P. Biochemical markers of bone turnover in diabetes patients--a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos. Int. 2014, 25, 1697–1708.
- Leite Duarte, M.E.; da Silva, R.D. Histomorphometric analysis of the bone tissue in patients with non-insulin-dependent diabetes (DMNID). Rev. Do Hosp. Das Clin. 1996, 51, 7–11.
- Premaor, M.; Parker, R.A.; Cummings, S.; Ensrud, K.; Cauley, J.A.; Lui, L.-Y.; Hillier, T.; Compston, J. Study of Osteoporotic Fractures (SOF) Research Group Predictive value of FRAX for fracture in obese older women. J. Bone Miner. Res. 2013, 28, 188–195.
- Leslie, W.D.; Orwoll, E.S.; Nielson, C.M.; Morin, S.N.; Majumdar, S.R.; Johansson, H.; Odén, A.; McCloskey, E.V.; Kanis, J.A. Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J. Bone Miner. Res. 2014, 29, 2511–2519.
- Giangregorio, L.M.; Leslie, W.D.; Lix, L.M.; Johansson, H.; Oden, A.; McCloskey, E.; Kanis, J.A. FRAX underestimates fracture risk in patients with diabetes. J. Bone Miner. Res. 2012, 27, 301–308.
- Langlois, J.A.; Mussolino, M.E.; Visser, M.; Looker, A.C.; Harris, T.; Madans, J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: The NHANES I epidemiologic follow-up study. Osteoporos. Int. 2001, 12, 763–768.
- Ensrud, K.E.; Fullman, R.L.; Barrett-Connor, E.; Cauley, J.A.; Stefanick, M.L.; Fink, H.A.; Lewis, C.E.; Orwoll, E. Osteoporotic Fractures in Men Study Research Group Voluntary weight reduction in older men increases hip bone loss: The osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2005, 90, 1998–2004.
- Bleicher, K.; Cumming, R.G.; Naganathan, V.; Travison, T.G.; Sambrook, P.N.; Blyth, F.M.; Handelsman, D.J.; Le Couteur, D.G.; Waite, L.M.; Creasey, H.M.; et al. The role of fat and lean mass in bone loss in older men: Findings from the CHAMP study. Bone 2011, 49, 1299–1305.
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am. J. Med. 1993, 95, 131–140.
- Salamone, L.M.; Cauley, J.A.; Black, D.M.; Simkin-Silverman, L.; Lang, W.; Gregg, E.; Palermo, L.; Epstein, R.S.; Kuller, L.H.; Wing, R. Effect of a lifestyle intervention on bone mineral density in premenopausal women: A randomized trial. Am. J. Clin. Nutr. 1999, 70, 97–103.
- Bakhireva, L.N.; Barrett-Connor, E.; Kritz-Silverstein, D.; Morton, D.J. Modifiable predictors of bone loss in older men: A prospective study. Am. J. Prev. Med. 2004, 26, 436–442.
- Crandall, C.J.; Yildiz, V.O.; Wactawski-Wende, J.; Johnson, K.C.; Chen, Z.; Going, S.B.; Wright, N.C.; Cauley, J.A. Postmenopausal weight change and incidence of fracture: Post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ 2015, 350, h25.
- Pop, L.C.; Sukumar, D.; Tomaino, K.; Schlussel, Y.; Schneider, S.H.; Gordon, C.L.; Wang, X.; Shapses, S.A. Moderate weight loss in obese and overweight men preserves bone quality. Am. J. Clin. Nutr. 2015, 101, 659–667.
- Seimon, R.V.; Wild-Taylor, A.L.; Keating, S.E.; McClintock, S.; Harper, C.; Gibson, A.A.; Johnson, N.A.; Fernando, H.A.; Markovic, T.P.; Center, J.R.; et al. Effect of Weight Loss via Severe vs Moderate Energy Restriction on Lean Mass and Body Composition Among Postmenopausal Women With Obesity: The TEMPO Diet Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1913733.
- Villalon, K.L.; Gozansky, W.S.; Van Pelt, R.E.; Wolfe, P.; Jankowski, C.M.; Schwartz, R.S.; Kohrt, W.M. A losing battle: Weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity 2011, 19, 2345–2350.
- Shah, K.; Armamento-Villareal, R.; Parimi, N.; Chode, S.; Sinacore, D.R.; Hilton, T.N.; Napoli, N.; Qualls, C.; Villareal, D.T. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J. Bone Miner. Res. 2011, 26, 2851–2859.
- Daly, R.M.; Dunstan, D.W.; Owen, N.; Jolley, D.; Shaw, J.E.; Zimmet, P.Z. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos. Int. 2005, 16, 1703–1712.
- Labouesse, M.A.; Gertz, E.R.; Piccolo, B.D.; Souza, E.C.; Schuster, G.U.; Witbracht, M.G.; Woodhouse, L.R.; Adams, S.H.; Keim, N.L.; Van Loan, M.D. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone 2014, 64, 138–146.
- Zernicke, R.F.; Salem, G.J.; Barnard, R.J.; Schramm, E. Long-term, high-fat-sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content, and mechanical properties. Bone 1995, 16, 25–31.
- Demigné, C.; Bloch-Faure, M.; Picard, N.; Sabboh, H.; Besson, C.; Rémésy, C.; Geoffroy, V.; Gaston, A.-T.; Nicoletti, A.; Hagège, A.; et al. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur. J. Nutr. 2006, 45, 298–306.
- Graham, L.S.; Tintut, Y.; Parhami, F.; Kitchen, C.M.R.; Ivanov, Y.; Tetradis, S.; Effros, R.B. Bone density and hyperlipidemia: The T-lymphocyte connection. J. Bone Miner. Res. 2010, 25, 2460–2469.
- Wang, Y.; Dellatore, P.; Douard, V.; Qin, L.; Watford, M.; Ferraris, R.P.; Lin, T.; Shapses, S.A. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr. Res. 2016, 36, 742–750.
- Lorincz, C.; Reimer, R.A.; Boyd, S.K.; Zernicke, R.F. High-fat, sucrose diet impairs geometrical and mechanical properties of cortical bone in mice. Br. J. Nutr. 2010, 103, 1302–1308.
- Zernicke, R.F.; Salem, G.J.; Barnard, R.J.; Woodward, J.S.; Meduski, J.W.; Meduski, J.D. Adaptations of immature trabecular bone to exercise and augmented dietary protein. Med. Sci. Sports Exerc. 1995, 27, 1486–1493.
- Tsanzi, E.; Light, H.R.; Tou, J.C. The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone 2008, 42, 960–968.
- Palermo, A.; Tuccinardi, D.; Defeudis, G.; Watanabe, M.; D’Onofrio, L.; Lauria Pantano, A.; Napoli, N.; Pozzilli, P.; Manfrini, S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int. J. Environ. Res. Public Health 2016, 13, 544.
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. Bmj 2010, 341, c3691.
