Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 3 by Rita Xu.
Approximately 60% of patients with tuberculosis (TB) have a cardiovascular disease, the most common associated pathological entities being pericarditis, myocarditis, and coronary artery disease.
- tuberculosis
- cardiovascular involvement
- pericarditis
1. Mycobacterium tuberculosis—An Old but “Still Standing” Enemy
Tuberculosis (TB) is a public health problem that, despite sustained global efforts, claimed 1.3 million deaths globally in 2020, with an upward trend to 1.4 million people in 2021. Difficulties in diagnosing and treating TB cases during the COVID-19 pandemic reversed years of progress in the field [1][2]. According to the WHO 2022 Incidence Report, about 10.6 million people became ill with TB in 2021, with immunocompromised patients accounting for about 7% of the total [3]. Epidemiological data published for the year 2021 show a higher infection rate among men compared to women [4]. Historical records refer to genus Mycobacterium as having existed over 150 million years ago [5]. Mentions of this infection are also found in the Old Testament, when Egypt was known as a geographical area with an extremely high prevalence of this pathology referred to at that time as ʺconsumptionʺ (from the Latin consumptio) [6][7][8][9]. Responsible for the appearance of severe pulmonary and extrapulmonary infections, Mycobacterium tuberculosis (MTB) has over time been attributed various names such as "schachepheth” [10], “phthisis”, or “king’s evil’ [11][12]
Although primarily a lung disease, extra-pulmonary TB can affect any organ or system. Of these, cardiovascular complications associated with disease or drug toxicity significantly worsen the prognosis [13]. Cardiovascular involvement primarily affects the pericardium, and only in very rare cases the myocardium or other cardiac structures. TB-associated pericarditis occurs frequently in immunocompromised patients associated with HIV infection, and less frequently in immunocompetent patients. In rare cases, whether associated with pericarditis or not, left ventricular systolic dysfunction occurs; the symptoms of heart failure may be overlooked as they overlap the general symptoms of TB [14][15].
2. Cardiovascular Involvement in TB
The cardiovascular structures most involved in tuberculosis are the pericardium, myocardium, and aorta (Figure 1).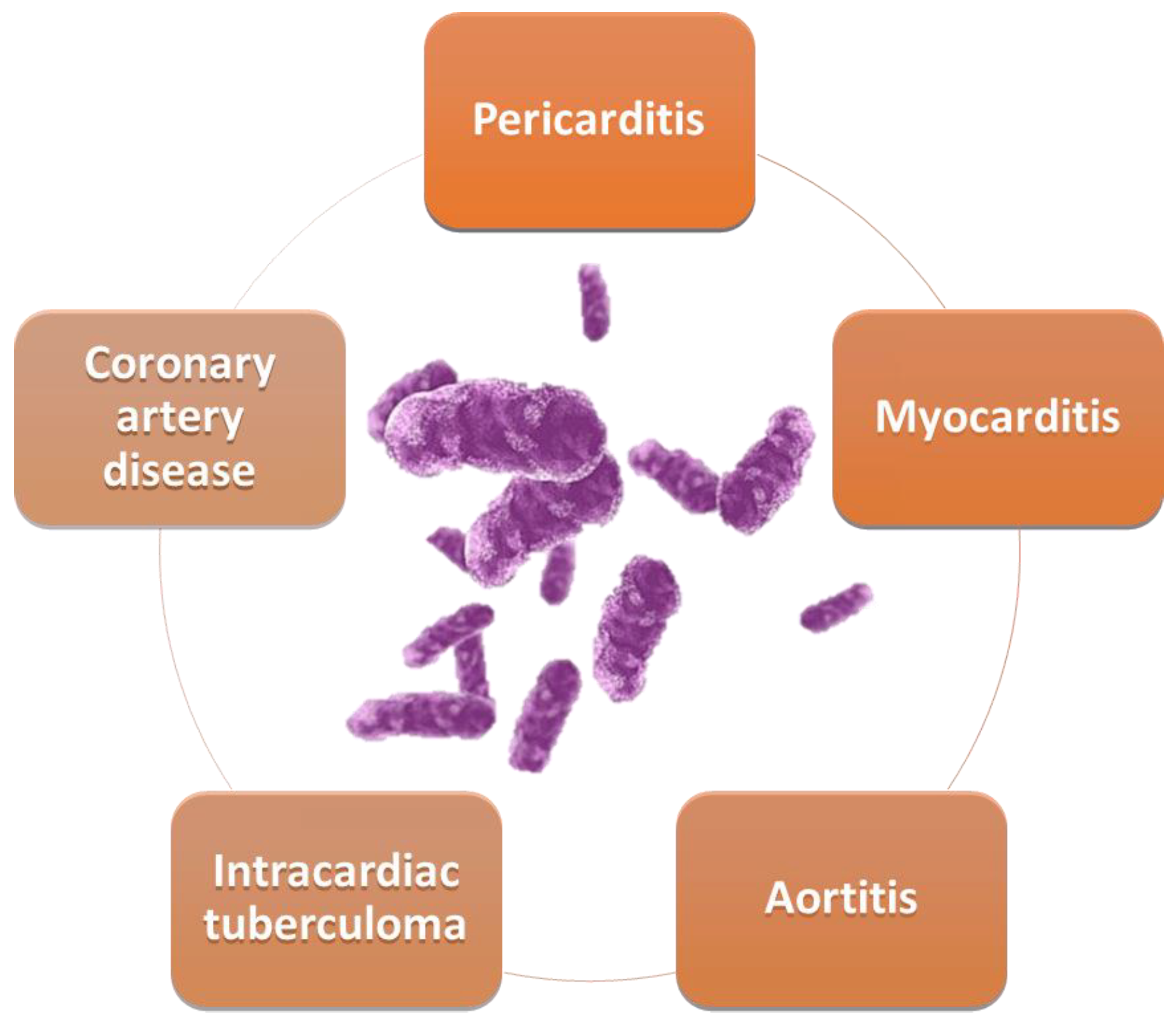
Figure 1. Cardiovascular involvement in TB.
2.1. Tuberculous Myocarditis
Myocardial involvement may be associated with pericarditis in the form of myopericarditis, or it may cover other clinical scenarios. Tuberculous myocarditis is particularly rare, with an estimated prevalence of less than 2% [16][17]. The first two cases of myocardial damage in TB were reported in 1664 by Maurocadat and in 1761 by Morgagni [18]. Epidemiological studies show a predominance of tuberculous myocarditis in patients under 45 years old, and it is twice as common in male patients [15]. Invasion of the myocardium by MTB is realized via the hematological route, by retrograde lymphatic insemination from the mediastinal nodes, or by direct invasion from the pericardium [19][20][21]. The different effects of MTB on the pericardium and myocardium can be explained on the one hand by the continuous movement of the myocardium, which indirectly prevents the lodging of bacilli, and on the other hand by the lactic acid produced, which has a protective role against bacilli [18][22]. Most cases have been reported in immunocompromised patients, who frequently have an HIV infection. Reported cases have frequently affected the left heart, and especially the left ventricle. Predominant right mediastinal lymph node involvement has been observed in many patients with MTB-induced myocarditis, which increases the risk of right heart damage by contiguity [23]. Myocardial damage is frequently asymptomatic, sometimes with severe consequences, leading to forms of acute heart failure [24][25]. In these cases, involvement of the right mediastinal lymph nodes has been observed, with a greater chance of contiguous involvement of the right side of the heart [15]. Identified forms include nodular myocardial damage with central caseation, miliary forms, or diffuse, inflammatory, giant cell forms [26]. A significant percentage of patients with myocardial damage also have pericarditis, which further worsens the prognosis [15]. Studies presenting data from endomyocardial biopsies indicate predominantly biventricular involvement in about 70% of cases, with isolated right ventricular dysfunction occurring in a small percentage of cases, only 8% [27]. The symptomatology of patients with myocarditis secondary to TB is variable, ranging from no symptoms to severe forms presenting at onset, with ventricular arrhythmias [28], sudden death, long QT syndrome [29], atrioventricular blocks, or clinical signs of congestive heart failure [24][30][31]. The clinical picture of these patients includes in some cases electrolyte imbalances, one of the most frequently reported being hypercalcemia [32]. The diagnostic criteria for myocarditis are the classic ones, represented by the identification of a high titer of myocardial enzymes together with the echocardiographic presence of left ventricular systolic dysfunction [15][19][33]. Nuclear magnetic resonance is an essential investigation in patients with myocarditis, highlighting in the T2 sequence a central and peripheral hypointense signal as well as a hyperintense thin line [34][35][36][37]. There are few data reported in the literature on the treatment of patients with tuberculous myocarditis, with clinical trials recommending the initiation of etiologic treatment. Improvement in symptoms does not eliminate the associated risk of sudden death, so these patients require regular monitoring, most often by multidisciplinary teams [38][39]. The most common complications reported were atrial fibrillation and sudden cardiac death [40]. Fulminant forms of MTB myocarditis can have an unfavorable outcome, especially in immunocompromised patients. Clinical studies report that 80% of fatal cases occur in female patients with associated LV systolic dysfunction [15]. Cases of chronic heart failure with preserved systolic function due to extensive intra-myocardial calcifications associated with latent MTB infection have also been reported [41].2.2. Coronary Artery Disease and Tuberculosis
One interaction being considered is that between TB and coronary atherosclerosis, the presence of TB being associated with a 1.76-fold increased risk of developing coronary artery disease [42][43]. Implicitly, patients with TB have an associated risk of acute myocardial infarction of 1.98 compared to a similar cohort of patients without TB [44][45][46]. Both TB and ischemic coronary artery disease are common in developing countries and their association is all the more frequent. Several mechanisms are thought to be behind this. A first mechanism is related to a chronic inflammatory reaction, cell-mediated immune activation with the release of cytokines and chemokines, following latent infection. A second mechanism is the initiation of an autoimmune process following chronic infection, with production of antibodies against mycobacterial heat shock protein-65 (HSP65) [47]. This causes an induced cross-reaction with human HSP65, leading to endothelial injury and stimulating atherogenesis. Heat shock proteins are a homogeneous group of proteins that arise in response to stress factors, originally discovered as a reaction to heat, hence the name. They show a high homogeneity between species and have in particular a chaperone role, but also mediate immune reactivity in certain diseases [48]. Animal model studies have shown that HSP65 inhibition affects IL-10 and paraoxonase-1 activity, while interferon-γ expression, myeloperoxidase activity, and the high-density lipoprotein inflammatory index tend to increase, leading to generalized as well as aortic atherosclerosis [49]. It has been observed that latent infection also results in elevated levels of interferon-γ, which may be a good predictor of progression to clinically manifest disease [50][51]. Moreover, considering that HSP65 mediates the early stages of the atherogenesis process, it is also being studied for the development of an anti-atherosclerotic vaccine [52]. A population-based study of more than 10,000 patients showed a 1.4-fold increased risk of acute coronary syndrome in patients diagnosed with TB compared to the general population [53]. This effect may be related to a combination of factors, with lung inflammation in general presumed to induce systemic inflammatory response, endothelial dysfunction, and atheroma plaque destabilization [54]. Recent data on latent TB have shown a high prevalence of ischemic coronary artery disease among these patients. Even in the absence of clinically manifest TB, chronic immune response to MBT can intensify the atherosclerotic process [15]. Another recent study showed a twofold increased likelihood of association of latent TB with acute myocardial infarction, after correcting for classical cardiovascular risk factors and other confounders [45]. Furthermore, it appears that vascular damage is not limited to the coronary arteries, and an association of latent TB with both peripheral arterial disease and ischemic stroke has been observed [55][56]. C-reactive protein (CRP), total white blood cell count, and neutrophil-to-lymphocyte ratio are three independent inflammatory predictors associated with a negative prognosis in coronary artery disease [57][58]. Serum CRP correlates with MTB bacterial load in sputum, having prognostic value and being associated with a high risk of death [59][60][61]. The beneficial effect of statins in reducing associated cardiovascular risk and decreasing systemic inflammation has been demonstrated, but its modulatory role in combination with MTB has not been fully elucidated to date [62][63][64][65][66]. There is a causal relationship between cholesterol and MTB, the bacterial agent needing cholesterol for infection and survival, with the caveat that the progression of infection is correlated with the ability of the immune system to limit infection [67][68]. Oxidized low-density lipoprotein plays an important role in patients with type 2 diabetes mellitus and TB, playing a central role in the formation of lipid-loaded foamy macrophages that contribute to the progression of tuberculous granulomas through lysosomal dysfunction [69][70][71]. Thus, various preclinical studies are reported in the literature in which statin administration is associated with stimulation of autophagy and phagosome maturation of MTB-infected macrophages. Research in murine models also highlights the beneficial role of statin administration in enhancing the therapeutic effect of first-line antituberculous drugs [72][73][74][75]. Statins have a beneficial role in the treatment of patients with TB infection and can be used as an adjuvant medication to standard treatment [67][76][77][78]. Administration of this hyperlipidemic medication increases cell resistance to MTB, but further clinical trials are needed in this research direction [76][79][80][81][82].2.3. Tuberculous Pericarditis
Tuberculous etiology of pericarditis is one of the most common along with the neoplastic etiology [83], with a prevalence depending on a country’s level of development [84]. Approximately 1–2% of TB patients have associated pericarditis [85]. This type of pericarditis is characterized by a significant inflammatory status [86], chronicity [85], and a high risk of progression to a constrictive form [87]. Pericardial insemination with MTB occurs retrogradely, by the lymphatic route, by hematological dissemination, or in rare cases by direct damage to surrounding structures such as the lungs, pleura, or spine [88]. In the case of HIV co-infection, the pathway of dissemination is hematological [89]. The most common form of presentation of tuberculous pericarditis is the effusive form (in about 80% of cases), the constrictive form being considered one of the most common sequelae [90]. The prevalence of the constrictive form of pericarditis is reduced in patients without TB [84]. In patients infected with MTB, this form of the disease is seen in 25% of cases, but this percentage may be higher than for other forms such as idiopathic or viral [91]. Some patients have an atypical clinical form that poses problems of diagnosis and treatment, often delaying the latter and thus worsening the prognosis of patients. Tuberculous pericarditis presents four distinct stages, with a clinical picture and imaging features specific to pathophysiological processes (Figure 2) [13].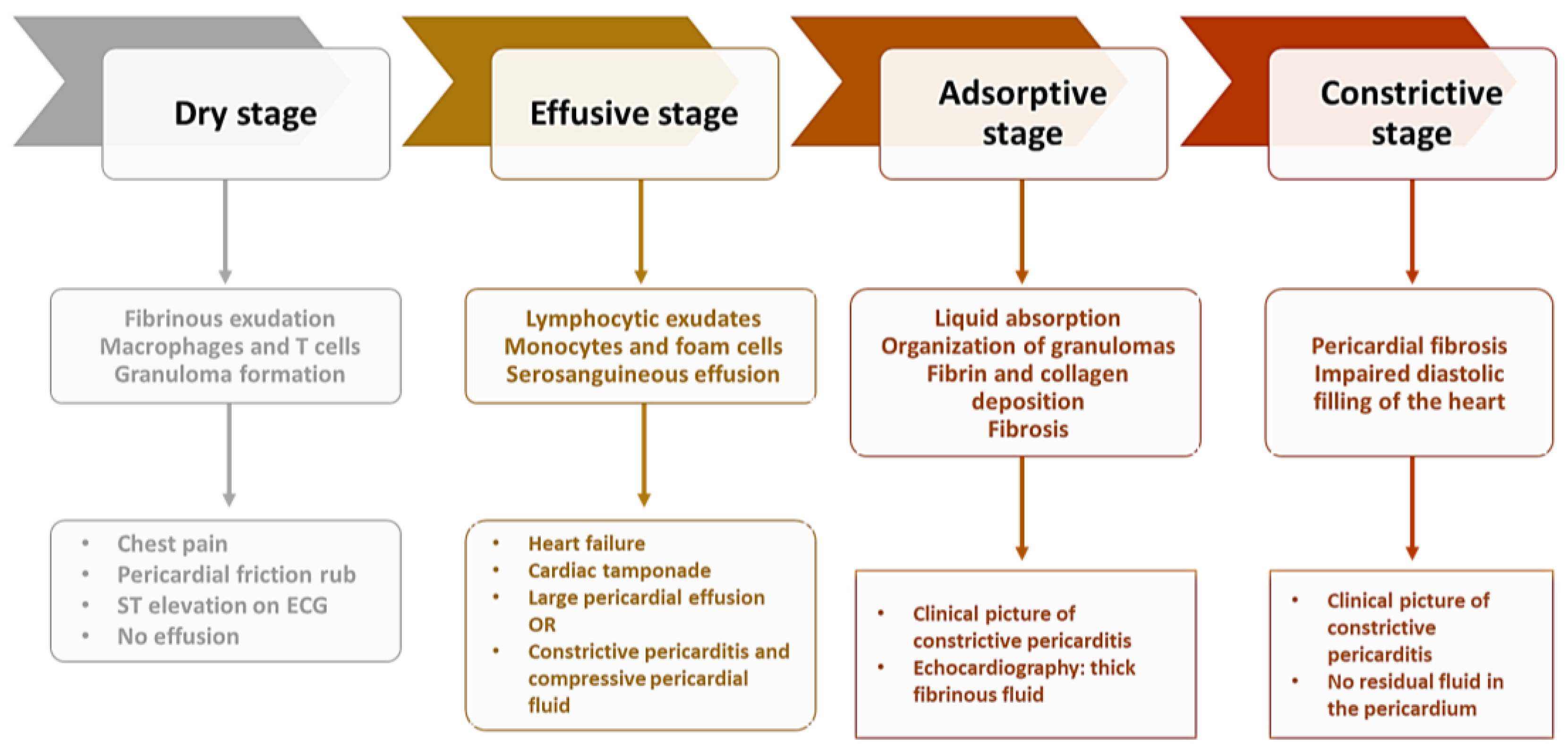
Figure 2. Stages of constrictive pericarditis.
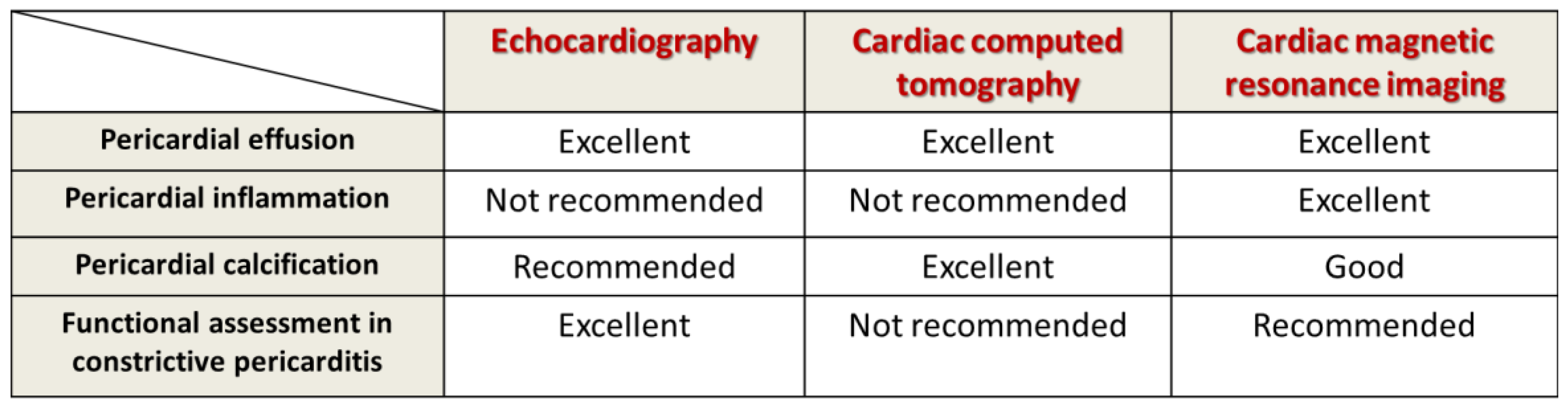
Figure 3. Multimodal imaging evaluation in tuberculous pericarditis.
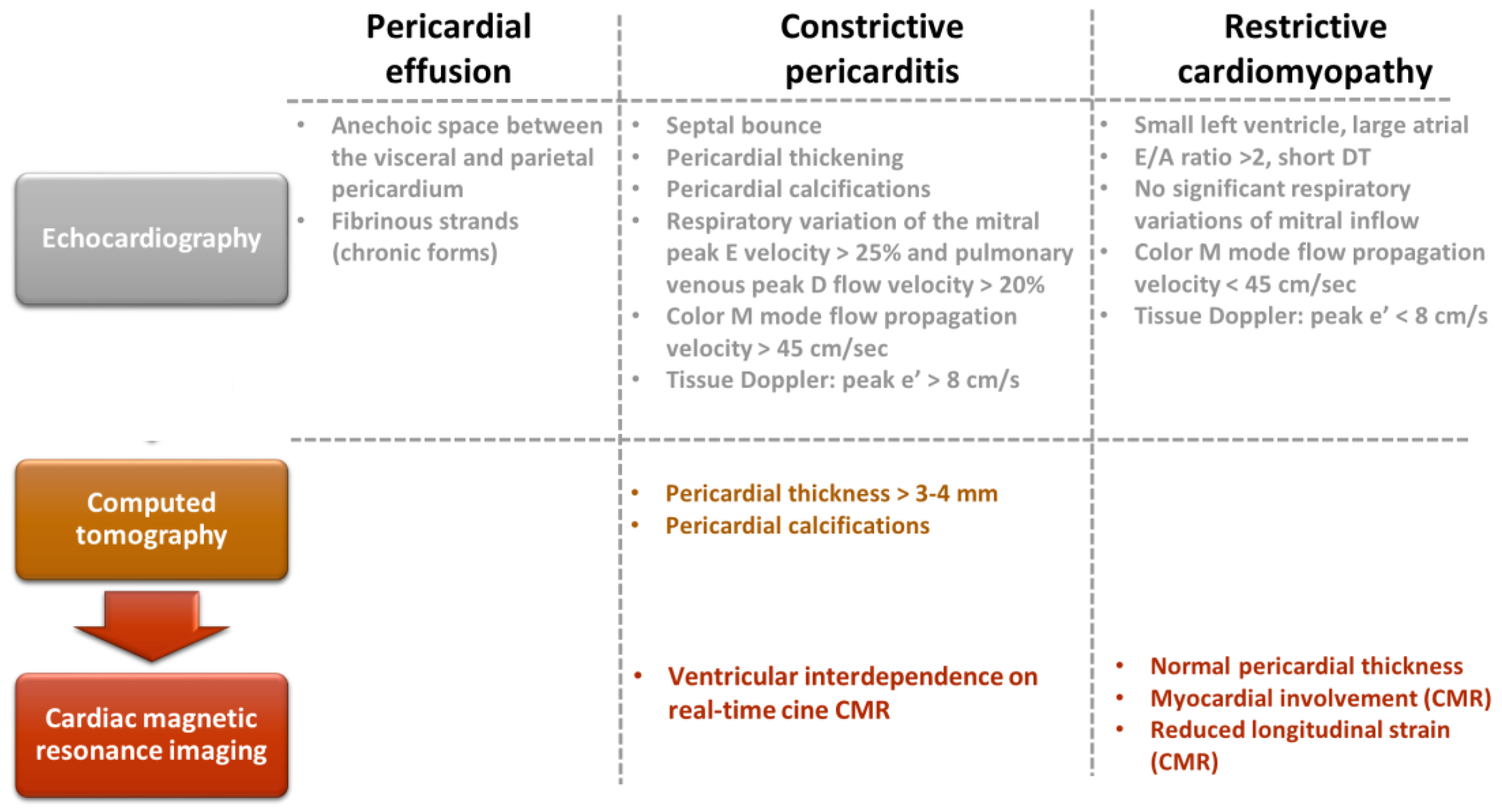
Figure 4. Multimodal imaging evaluation of patients with pericardial effusion, constrictive pericarditis, and restrictive cardiomyopathy.
References
- 2.2 TB Mortality. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021/disease-burden/mortality (accessed on 27 November 2022).
- 2.2 TB Mortality. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden/2-2-tb-mortality (accessed on 27 November 2022).
- 2.1 TB Incidence. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden/2-1-tb-incidence (accessed on 27 November 2022).
- Global Tuberculosis Report. 2021. Available online: https://www.who.int/publications-detail-redirect/9789240037021 (accessed on 29 November 2022).
- Hayman, J. Mycobacterium Ulcerans: An Infection From Jurassic Time? The Lancet 1984, 324, 1015–1016.
- Simpson, D.P. Cassell’s Latin Dictionary; Macmillan: New York, NY, USA, 1977.
- Brothwell, D. Diseases in Antiquity: A Survey of the Diseases, Injuries, and Surgery of Early Populations, 1st, ed.; Charles C Thomas Pub, Ltd.: Springfield, IL, USA, 1967; ISBN 978-0-398-00233-6.
- Morse, D.; Brothwell, D.R.; Ucko, P.J. Tuberculosis in Ancient Egypt. Am. Rev. Respir. Dis. 1964, 90, 524–541.
- Cave, A.J.E.; Demonstrator, A. The Evidence for the Incidence of Tuberculosis in Ancient Egypt. Br. J. Tuberc. 1939, 33, 142–152.
- Strong, J. Strong’s Exhaustive Concordance of the Bible; Hendrickson Publishers: Peabody, MA, USA, 2009; ISBN 978-1-59856-378-8.
- Daniel, V.S.; Daniel, T.M. Old Testament Biblical References to Tuberculosis. Clin. Infect. Dis. 1999, 29, 1557–1558.
- Murray, J.F.; Rieder, H.L.; Finley-Croswhite, A. The King’s Evil and the Royal Touch: The Medical History of Scrofula. Int. J. Tuberc. Lung Dis. 2016, 20, 713–716.
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A. The Neglected Tropical Diseases, Other Infectious Diseases Affecting the Heart (the NET-Heart Project) Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435.
- Mutyaba, A.K.; Ntsekhe, M. Tuberculosis and the Heart. Cardiol. Clin. 2017, 35, 135–144.
- Michira, B.N.; Alkizim, F.O.; Matheka, D.M. Patterns and Clinical Manifestations of Tuberculous Myocarditis: A Systematic Review of Cases. Pan Afr. Med. J. 2015, 21, 118.
- Das, K.M.; Mansoori, T.A.; Alattar, Y.H.; Gorkom, K.V.; Shamisi, A.; Melethil, A.P.; Alkoteesh, J.A. Tuberculosis of the Heart: A Diagnostic Challenge. Tomography 2022, 8, 1649–1665.
- Patil, S.V.; Toshniwal, S.; Gondhali, G.; Patil, D. Pulmonary Tuberculosis with Cardiac Dysfunction: An Ignored Combination! Electron. J. Gen. Med. 2023, 20, em437.
- Awasthy, N.; Garg, R.; Goel, A.; Bhatia, M.; Radhakrishnan, S. Ventricular Arrhythmia: A Feature of Tubercular Myocarditis. Ann. Pediatr. Cardiol. 2019, 12, 53.
- Choudhary, N.; Abera, H.; Naik, R.B. Tuberculosis Presenting With Acute Myocarditis and Systolic Heart Failure. Cureus 2021, 13, e13229.
- Wallis, P.J.; Branfoot, A.C.; Emerson, P.A. Sudden Death Due to Myocardial Tuberculosis. Thorax 1984, 39, 155–156.
- Kirsten, K.; Schaedel, C. Sudden Death Due to Myocardial Tuberculosis. Thorax 1985, 40, 799.
- du Toit-Prinsloo, L.; Saayman, G. “Death at the Wheel” Due to Tuberculosis of the Myocardium: A Case Report. Cardiovasc. Pathol. 2016, 25, 271–274.
- Maeder, M.; Ammann, P.; Rickli, H.; Schoch, O.D. Fever and Night Sweats in a 22-Year-Old Man with a Mediastinal Mass Involving the Heart. Chest 2003, 124, 2006–2009.
- Raffali, M.A.; Muhammad, S.F.; Tiau Wei Jyung, P.; Farouk, D.; Zohdi, A.; Che Hassan, H.H. Disseminated Tuberculosis With Myocarditis and Intracardiac Thrombus in a Previously Young Healthy Woman. JACC Case Rep. 2021, 3, 1661–1666.
- Al-Jahdali Tuberculous Myocarditis Is Not Always Fatal: Report of Three Confirmed Cases with Uneventful Outcome. Available online: https://www.ijmyco.org/article.asp?issn=2212-5531;year=2017;volume=6;issue=1;spage=111;epage=115;aulast=Al-Jahdali (accessed on 30 November 2022).
- Gautam, M.P.; Sogunuru, G.; Subramanyam, G.; Viswanath, R.C. Tuberculous Myocarditis Presenting as a Refractory Ventricular Tachycardia of Biventricular Origin. J. Coll. Med. Sci.-Nepal 2011, 7, 60–66.
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative Evaluation of Left and Right Ventricular Endomyocardial Biopsy: Differences in Complication Rate and Diagnostic Performance. Circulation 2010, 122, 900–909.
- O’Neill, P.G.; Rokey, R.; Greenberg, S.; Pacifico, A. Resolution of Ventricular Tachycardia and Endocardial Tuberculoma Following Antituberculosis Therapy. Chest 1991, 100, 1467–1469.
- Díaz-Peromingo, J.A.; Mariño-Callejo, A.I.; González-González, C.; García-Rodríguez, J.F.; Ameneiros-Lago, M.E.; Sesma-Sánchez, P. Tuberculous Myocarditis Presenting as Long QT Syndrome. Eur. J. Intern. Med. 2000, 11, 340–342.
- Vennamaneni, V.; Chohan, F.; Rad, P.; Rodriguez, J.; Gupta, R.; Michel, G. Clinical Presentation of a Patient With Tuberculous Myocarditis: Case Report and Review of Literature. Cureus 2022, 14, e22715.
- Kapoor, O.P.; Mascarenhas, E.; Rananaware, M.M.; Gadgil, R.K. Tuberculoma of the Heart. Report of 9 Cases. Am. Heart J. 1973, 86, 334–340.
- Alkhuja, S.; Miller, A. Tuberculosis and Sudden Death: A Case Report and Review. Heart Lung J. Crit. Care 2001, 30, 388–391.
- Ren, M.; Zhang, C.; Zhang, X.; Zhong, J. Acute Tuberculous Myopericarditis Mimicking Acute Myocardial Infarction: A Case Report and Literature Review. Exp. Ther. Med. 2016, 11, 2373–2378.
- Breton, G.; Leclerc, S.; Longuet, P.; Leport, C.; Vildé, J.-L.; Laissy, J.-P. Myocardial localisation of tuberculosis: The diagnostic value of cardiac MRI. Presse Medicale Paris Fr. 1983 2005, 34, 293–296.
- Rajeshwari, K.; Gupta, S.; Dubey, A.; Gera, R. Asymptomatic Multiple Intracardiac Tuberculomas in a Child. Cardiol. J. 2012, 19, 518–520.
- Lambatten, D.; Hammi, S.; Rhofir, Y.; Bourkadi, J.E. Myocardial tuberculoma: Unusual location of tuberculosis: A new observation and review of the literature. Pan Afr. Med. J. 2016, 24, 32.
- Dixit, R.; Chowdhury, V.; Singh, S. Case Report: Myocardial Tuberculosis-MRI. Indian J. Radiol. Imaging 2009, 19, 57–59.
- Amonkar, G.; Rupani, A.; Shah, V.; Parmar, H. Sudden Death in Tuberculous Myocarditis. Cardiovasc. Pathol. 2009, 18, 247–248.
- Kumar, P.; Sharma, S.; Bisht, D.; Panwar, R. Tuberculous Dilated Cardiomyopathy with Myocarditis. Indian J. Pediatr. 2020, 87, 161.
- Dada, M.A.; Lazarus, N.G.; Kharsany, A.B.; Sturm, A.W. Sudden Death Caused by Myocardial Tuberculosis: Case Report and Review of the Literature. Am. J. Forensic Med. Pathol. 2000, 21, 385–388.
- Hitsumoto, T.; Ikeda, S.; Matsukage, S.; Hamada, M. Extensive Myocardial Calcinosis Due to Mycobacterium tuberculosis. Eur. Heart J. 2016, 37, 1195.
- Wongtrakul, W.; Charoenngam, N.; Ungprasert, P. Tuberculosis and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis. Indian J. Tuberc. 2020, 67, 182–188.
- Basham, C.A.; Smith, S.J.; Romanowski, K.; Johnston, J.C. Cardiovascular Morbidity and Mortality among Persons Diagnosed with Tuberculosis: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235821.
- Huaman, M.A.; Kryscio, R.J.; Fichtenbaum, C.J.; Henson, D.; Salt, E.; Sterling, T.R.; Garvy, B.A. Tuberculosis and Risk of Acute Myocardial Infarction: A Propensity Score-Matched Analysis. Epidemiol. Infect. 2017, 145, 1363–1367.
- Huaman, M.A.; Ticona, E.; Miranda, G.; Kryscio, R.J.; Mugruza, R.; Aranda, E.; Rondan, P.L.; Henson, D.; Ticona, C.; Sterling, T.R.; et al. The Relationship Between Latent Tuberculosis Infection and Acute Myocardial Infarction. Clin. Infect. Dis. 2018, 66, 886–892.
- Salindri, A.D.; Wang, J.-Y.; Lin, H.-H.; Magee, M.J. Post-Tuberculosis Incidence of Diabetes, Myocardial Infarction, and Stroke: Retrospective Cohort Analysis of Patients Formerly Treated for Tuberculosis in Taiwan, 2002-2013. Int. J. Infect. Dis. 2019, 84, 127–130.
- Khoufi, E.A.A. Association between Latent Tuberculosis and Ischemic Heart Disease: A Hospital-Based Cross-Sectional Study from Saudi Arabia. Pan Afr. Med. J. 2021, 38, 362.
- Rodríguez-Iturbe, B.; Johnson, R. Heat Shock Proteins and Cardiovascular Disease. Physiol. Int. 2018, 105, 19–37.
- Sun, H.; Shen, J.; Liu, T.; Tan, Y.; Tian, D.; Luo, T.; Lai, W.; Dai, M.; Guo, Z. Heat Shock Protein 65 Promotes Atherosclerosis through Impairing the Properties of High Density Lipoprotein. Atherosclerosis 2014, 237, 853–861.
- Huaman, M.A.; Deepe, G.S.; Fichtenbaum, C.J. Elevated Circulating Concentrations of Interferon-Gamma in Latent Tuberculosis Infection. Pathog. Immun. 2016, 1, 291–303.
- Zak, D.E.; Penn-Nicholson, A.; Scriba, T.J.; Thompson, E.; Suliman, S.; Amon, L.M.; Mahomed, H.; Erasmus, M.; Whatney, W.; Hussey, G.D.; et al. A Prospective Blood RNA Signature for Tuberculosis Disease Risk. Lancet Lond. Engl. 2016, 387, 2312–2322.
- Grundtman, C.; Jakic, B.; Buszko, M.; Onestingel, E.; Almanzar, G.; Demetz, E.; Dietrich, H.; Cappellano, G.; Wick, G. Mycobacterial Heat Shock Protein 65 (MbHSP65)-Induced Atherosclerosis: Preventive Oral Tolerization and Definition of Atheroprotective and Atherogenic MbHSP65 Peptides. Atherosclerosis 2015, 242, 303–310.
- Chung, W.-S.; Lin, C.-L.; Hung, C.-T.; Chu, Y.-H.; Sung, F.-C.; Kao, C.-H.; Yeh, J.-J. Tuberculosis Increases the Subsequent Risk of Acute Coronary Syndrome: A Nationwide Population-Based Cohort Study. Int. J. Tuberc. Lung Dis. 2014, 18, 79–83.
- Van Eeden, S.; Leipsic, J.; Paul Man, S.F.; Sin, D.D. The Relationship between Lung Inflammation and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 11–16.
- Sheu, J.-J.; Chiou, H.-Y.; Kang, J.-H.; Chen, Y.-H.; Lin, H.-C. Tuberculosis and the Risk of Ischemic Stroke: A 3-Year Follow-Up Study. Stroke 2010, 41, 244–249.
- Wang, S.-H.; Chien, W.-C.; Chung, C.-H.; Lin, F.-H.; Peng, C.-K.; Chian, C.-F.; Shen, C.-H. Tuberculosis Increases the Risk of Peripheral Arterial Disease: A Nationwide Population-Based Study: TB Increases the Risk of PAD. Respirology 2017, 22, 1670–1676.
- Liu, Y.; Jia, S.; Yao, Y.; Tang, X.-F.; Xu, N.; Jiang, L.; Gao, Z.; Chen, J.; Yang, Y.-J.; Gao, R.-L.; et al. Impact of High-Sensitivity C-Reactive Protein on Coronary Artery Disease Severity and Outcomes in Patients Undergoing Percutaneous Coronary Intervention. J. Cardiol. 2020, 75, 60–65.
- Chen, H.; Li, M.; Liu, L.; Dang, X.; Zhu, D.; Tian, G. Monocyte/Lymphocyte Ratio Is Related to the Severity of Coronary Artery Disease and Clinical Outcome in Patients with Non-ST-Elevation Myocardial Infarction. Medicine (Baltimore) 2019, 98, e16267.
- Mesquita, E.D.D.; Gil-Santana, L.; Ramalho, D.; Tonomura, E.; Silva, E.C.; Oliveira, M.M.; Andrade, B.B.; Kritski, A. For the Rede-TB Study group Associations between Systemic Inflammation, Mycobacterial Loads in Sputum and Radiological Improvement after Treatment Initiation in Pulmonary TB Patients from Brazil: A Prospective Cohort Study. BMC Infect. Dis. 2016, 16, 368.
- Wilson, D.; Moosa, M.-Y.S.; Cohen, T.; Cudahy, P.; Aldous, C.; Maartens, G. Evaluation of Tuberculosis Treatment Response With Serial C-Reactive Protein Measurements. Open Forum Infect. Dis. 2018, 5, ofy253.
- Cubillos-Angulo, J.M.; Nogueira, B.M.F.; Arriaga, M.B.; Barreto-Duarte, B.; Araújo-Pereira, M.; Fernandes, C.D.; Vinhaes, C.L.; Villalva-Serra, K.; Nunes, V.M.; Miguez-Pinto, J.P.; et al. Host-Directed Therapies in Pulmonary Tuberculosis: Updates on Anti-Inflammatory Drugs. Front. Med. 2022, 9, 970408.
- Jain, M.K.; Ridker, P.M. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987.
- Liberale, L.; Carbone, F.; Montecucco, F.; Sahebkar, A. Statins Reduce Vascular Inflammation in Atherogenesis: A Review of Underlying Molecular Mechanisms. Int. J. Biochem. Cell Biol. 2020, 122, 105735.
- Montecucco, F.; Mach, F. Update on Statin-Mediated Anti-Inflammatory Activities in Atherosclerosis. Semin. Immunopathol. 2009, 31, 127–142.
- Shahbaz, S.K.; Sadeghi, M.; Koushki, K.; Penson, P.E.; Sahebkar, A. Regulatory T Cells: Possible Mediators for the Anti-Inflammatory Action of Statins. Pharmacol. Res. 2019, 149, 104469.
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and Cons. Med. Clin. 2018, 150, 398–402.
- Guerra-De-Blas, P.D.C.; Torres-González, P.; Bobadilla-Del-Valle, M.; Sada-Ovalle, I.; Ponce-De-León-Garduño, A.; Sifuentes-Osornio, J. Potential Effect of Statins on Mycobacterium Tuberculosis Infection. J. Immunol. Res. 2018, 2018, 7617023.
- Tahir, F.; Bin Arif, T.; Ahmed, J.; Shah, S.R.; Khalid, M. Anti-Tuberculous Effects of Statin Therapy: A Review of Literature. Cureus 2020, 12, e7404.
- Vrieling, F.; Wilson, L.; Rensen, P.C.N.; Walzl, G.; Ottenhoff, T.H.M.; Joosten, S.A. Oxidized Low-Density Lipoprotein (OxLDL) Supports Mycobacterium Tuberculosis Survival in Macrophages by Inducing Lysosomal Dysfunction. PLoS Pathog. 2019, 15, e1007724.
- Shim, D.; Kim, H.; Shin, S.J. Mycobacterium Tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy. Front. Immunol. 2020, 11, 910.
- Park, H.-E.; Lee, W.; Choi, S.; Jung, M.; Shin, M.-K.; Shin, S.J. Modulating Macrophage Function to Reinforce Host Innate Resistance against Mycobacterium Avium Complex Infection. Front. Immunol. 2022, 13, 931876.
- Parihar, S.P.; Guler, R.; Khutlang, R.; Lang, D.M.; Hurdayal, R.; Mhlanga, M.M.; Suzuki, H.; Marais, A.D.; Brombacher, F. Statin Therapy Reduces the Mycobacterium Tuberculosis Burden in Human Macrophages and in Mice by Enhancing Autophagy and Phagosome Maturation. J. Infect. Dis. 2014, 209, 754–763.
- Bruiners, N.; Dutta, N.K.; Guerrini, V.; Salamon, H.; Yamaguchi, K.D.; Karakousis, P.C.; Gennaro, M.L. The Anti-Tubercular Activity of Simvastatin Is Mediated by Cholesterol-Driven Autophagy via the AMPK-MTORC1-TFEB Axis. J. Lipid Res. 2020, 61, 1617–1628.
- Dutta, N.K.; Bruiners, N.; Pinn, M.L.; Zimmerman, M.D.; Prideaux, B.; Dartois, V.; Gennaro, M.L.; Karakousis, P.C. Statin Adjunctive Therapy Shortens the Duration of TB Treatment in Mice. J. Antimicrob. Chemother. 2016, 71, 1570–1577.
- Dutta, N.K.; Bruiners, N.; Zimmerman, M.D.; Tan, S.; Dartois, V.; Gennaro, M.L.; Karakousis, P.C. Adjunctive Host-Directed Therapy With Statins Improves Tuberculosis-Related Outcomes in Mice. J. Infect. Dis. 2020, 221, 1079–1087.
- Li, X.; Sheng, L.; Lou, L. Statin Use May Be Associated With Reduced Active Tuberculosis Infection: A Meta-Analysis of Observational Studies. Front. Med. 2020, 7, 121.
- Guerra-De-Blas, P.D.C.; Bobadilla-Del-Valle, M.; Sada-Ovalle, I.; Estrada-García, I.; Torres-González, P.; López-Saavedra, A.; Guzmán-Beltrán, S.; Ponce-de-León, A.; Sifuentes-Osornio, J. Simvastatin Enhances the Immune Response Against Mycobacterium Tuberculosis. Front. Microbiol. 2019, 10, 2097.
- Chidambaram, V.; Zhou, L.; Ruelas Castillo, J.; Kumar, A.; Ayeh, S.K.; Gupte, A.; Wang, J.-Y.; Karakousis, P.C. Higher Serum Cholesterol Levels Are Associated With Reduced Systemic Inflammation and Mortality During Tuberculosis Treatment Independent of Body Mass Index. Front. Cardiovasc. Med. 2021, 8, 696517.
- Su, V.Y.-F.; Pan, S.-W.; Yen, Y.-F.; Feng, J.-Y.; Su, W.-J.; Chen, Y.-M. Statin Use and Impact on Tuberculosis Risk. Expert Rev. Anti Infect. Ther. 2021, 19, 1093–1098.
- Meregildo-Rodriguez, E.D.; Chunga-Chévez, E.V.; Gianmarco, R.-A.L.; Vásquez-Tirado, G.A. Further Insights into to the Role of Statins against Active Tuberculosis: Systematic Review and Meta-Analysis. Infez. Med. 2022, 30, 194–203.
- Duan, H.; Liu, T.; Zhang, X.; Yu, A.; Cao, Y. Statin Use and Risk of Tuberculosis: A Systemic Review of Observational Studies. Int. J. Infect. Dis. 2020, 93, 168–174.
- Chen, Y.-T.; Kuo, S.-C.; Chao, P.-W.; Chang, Y.-Y. Use of Lipid-Lowering Agents Is Not Associated with Improved Outcomes for Tuberculosis Patients on Standard-Course Therapy: A Population-Based Cohort Study. PLoS ONE 2019, 14, e0210479.
- Vakamudi, S.; Ho, N.; Cremer, P.C. Pericardial Effusions: Causes, Diagnosis, and Management. Prog. Cardiovasc. Dis. 2017, 59, 380–388.
- Ntsekhe, M.; Shey Wiysonge, C.; Commerford, P.J.; Mayosi, B.M. The Prevalence and Outcome of Effusive Constrictive Pericarditis: A Systematic Review of the Literature. Cardiovasc. J. Afr. 2012, 23, 281–285.
- Mayosi, B.M.; Burgess, L.J.; Doubell, A.F. Tuberculous Pericarditis. Circulation 2005, 112, 3608–3616.
- Reuter, H.; Burgess, L.J.; Carstens, M.E.; Doubell, A.F. Characterization of the Immunological Features of Tuberculous Pericardial Effusions in HIV Positive and HIV Negative Patients in Contrast with Non-Tuberculous Effusions. Tuberc. Edinb. Scotl. 2006, 86, 125–133.
- Yu, G.; Zhong, F.; Shen, Y.; Zheng, H. Diagnostic Accuracy of the Xpert MTB/RIF Assay for Tuberculous Pericarditis: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0257220.
- Spodick, D.H. Tuberculous Pericarditis. AMA Arch. Intern. Med. 1956, 98, 737–749.
- Ntsekhe, M.; Mayosi, B.M. Tuberculous Pericarditis with and without HIV. Heart Fail. Rev. 2013, 18, 367–373.
- Mayosi, B.M.; Wiysonge, C.S.; Ntsekhe, M.; Volmink, J.A.; Gumedze, F.; Maartens, G.; Aje, A.; Thomas, B.M.; Thomas, K.M.; Awotedu, A.A.; et al. Clinical Characteristics and Initial Management of Patients with Tuberculous Pericarditis in the HIV Era: The Investigation of the Management of Pericarditis in Africa (IMPI Africa) Registry. BMC Infect. Dis. 2006, 6, 2.
- Schrire, V. Experience with Pericarditis at Groote Schuur Hospital, Cape Town: An Analysis of One Hundred and Sixty Cases Studied over a Six-Year Period. S. Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 1959, 33, 810–817.
- Khorb, N.E.; Ouali, L.E.; Lahlou, I.; Ouaha, L.; Akoudad, H. La péricardite chronique constrictive. Moroc. J. Cardiol. 2012, 7, 16–20.
- Clare, G.C.; Troughton, R.W. Management of Constrictive Pericarditis in the 21st Century. Curr. Treat. Options Cardiovasc. Med. 2007, 9, 436–442.
- Ghavidel, A.A.; Gholampour, M.; Kyavar, M.; Mirmesdagh, Y.; Tabatabaie, M.-B. Constrictive Pericarditis Treated by Surgery. Tex. Heart Inst. J. 2012, 39, 199–205.
- Karima, T.; Nesrine, B.Z.; Hatem, L.; Skander, B.O.; Raouf, D.; Selim, C. Constrictive Pericarditis: 21 Years’ Experience and Review of Literature. Pan Afr. Med. J. 2021, 38, 141.
- Yamani, N.; Abbasi, A.; Almas, T.; Mookadam, F.; Unzek, S. Diagnosis, Treatment, and Management of Pericardial Effusion- Review. Ann. Med. Surg. 2022, 80, 104142.
- The EACVI Textbook of Echocardiography; Lancellotti, P.; Zamorano, J.L.; Habib, G.; Badano, L. (Eds.) Oxford University Press: Oxford, UK, 2016; Volume 1, ISBN 978-0-19-872601-2.
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964.
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.M.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic Properties of Chest Ultrasound in Thoracic Tuberculosis-A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2235.
- Cocco, G.; Boccatonda, A.; Rossi, I.; D’Ardes, D.; Corvino, A.; Delli Pizzi, A.; Ucciferri, C.; Katia, F.; Jacopo, V. Early Detection of Pleuro-Pulmonary Tuberculosis by Bedside Lung Ultrasound: A Case Report and Review of Literature. Clin. Case Rep. 2022, 10, e05739.
- Giannelli, F.; Cozzi, D.; Cavigli, E.; Campolmi, I.; Rinaldi, F.; Giachè, S.; Rogasi, P.G.; Miele, V.; Bartolucci, M. Lung Ultrasound (LUS) in Pulmonary Tuberculosis: Correlation with Chest CT and X-Ray Findings. J. Ultrasound 2022, 25, 625–634.
- Isiguzo, G.; Du Bruyn, E.; Howlett, P.; Ntsekhe, M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr. Cardiol. Rep. 2020, 22, 2.
- Noubiap, J.J.; Agbor, V.N.; Ndoadoumgue, A.L.; Nkeck, J.R.; Kamguia, A.; Nyaga, U.F.; Ntsekhe, M. Epidemiology of Pericardial Diseases in Africa: A Systematic Scoping Review. Heart Br. Card. Soc. 2019, 105, 180–188.
- Wang, S.; Wang, J.; Liu, J.; Zhang, Z.; He, J.; Wang, Y. A Case Report and Review of Literature: Tuberculous Pericarditis with Pericardial Effusion as the Only Clinical Manifestation. Front. Cardiovasc. Med. 2022, 9, 1020672.
- Ntsekhe, M.; Matthews, K.; Syed, F.F.; Deffur, A.; Badri, M.; Commerford, P.J.; Gersh, B.J.; Wilkinson, K.A.; Wilkinson, R.J.; Mayosi, B.M. Prevalence, Hemodynamics, and Cytokine Profile of Effusive-Constrictive Pericarditis in Patients with Tuberculous Pericardial Effusion. PLoS ONE 2013, 8, e77532.
- Minardi, M.L.; Fato, I.; Di Gennaro, F.; Mosti, S.; Mastrobattista, A.; Cerva, C.; Libertone, R.; Saracino, A.; Goletti, D.; Girardi, E.; et al. Common and Rare Hematological Manifestations and Adverse Drug Events during Treatment of Active TB: A State of Art. Microorganisms 2021, 9, 1477.
- Barzegari, S.; Afshari, M.; Movahednia, M.; Moosazadeh, M. Prevalence of Anemia among Patients with Tuberculosis: A Systematic Review and Meta-Analysis. Indian J. Tuberc. 2019, 66, 299–307.
- De Vita, E.; Segala, F.V.; Amone, J.; Samuel, K.; Marotta, C.; Putoto, G.; Nassali, R.; Lochoro, P.; Bavaro, D.F.; Ictho, J.; et al. Subacute Cardiac Tamponade Due to Tuberculous Pericarditis Diagnosed by Urine Lipoarabinomannan Assay in a Immunocompetent Patient in Oyam District, Uganda: A Case Report. Int. J. Environ. Res. Public. Health 2022, 19, 15143.
- Mathabire Rucker, S.C.; Cossa, L.; Harrison, R.E.; Mpunga, J.; Lobo, S.; Kisaka Kimupelenge, P.; Mandar Kol’Ampwe, F.; Amoros Quiles, I.; Molfino, L.; Szumilin, E.; et al. Feasibility of Using Determine TB-LAM to Diagnose Tuberculosis in HIV-Positive Patients in Programmatic Conditions: A Multisite Study. Glob. Health Action 2019, 12, 1672366.
- Pandie, S.; Peter, J.G.; Kerbelker, Z.S.; Meldau, R.; Theron, G.; Govender, U.; Ntsekhe, M.; Dheda, K.; Mayosi, B.M. The Diagnostic Accuracy of Pericardial and Urinary Lipoarabinomannan (LAM) Assays in Patients with Suspected Tuberculous Pericarditis. Sci. Rep. 2016, 6, 32924.
- Chatterjee, D.; Bozic, C.M.; McNeil, M.; Brennan, P.J. Structural Features of the Arabinan Component of the Lipoarabinomannan of Mycobacterium Tuberculosis. J. Biol. Chem. 1991, 266, 9652–9660.
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial Lipoarabinomannan and Related Lipoglycans: From Biogenesis to Modulation of the Immune Response: The Mycobacterial Lipoarabinomannan and Related Molecules. Mol. Microbiol. 2004, 53, 391–403.
- Mayosi, B.M.; Wiysonge, C.S.; Ntsekhe, M.; Gumedze, F.; Volmink, J.A.; Maartens, G.; Aje, A.; Thomas, B.M.; Thomas, K.M.; Awotedu, A.A.; et al. Mortality in Patients Treated for Tuberculous Pericarditis in Sub-Saharan Africa. S. Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 2008, 98, 36–40.
- Syed, F.F.; Mayosi, B.M. A Modern Approach to Tuberculous Pericarditis. Prog. Cardiovasc. Dis. 2007, 50, 218–236.
- Jung, I.Y.; Song, Y.G.; Choi, J.Y.; Kim, M.H.; Jeong, W.Y.; Oh, D.H.; Kim, Y.C.; Song, J.E.; Kim, E.J.; Lee, J.U.; et al. Predictive Factors for Unfavorable Outcomes of Tuberculous Pericarditis in Human Immunodeficiency Virus-Uninfected Patients in an Intermediate Tuberculosis Burden Country. BMC Infect. Dis. 2016, 16, 719.
More
