You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Felice Cinque.
HIV infection and nonalcoholic fatty liver disease (NAFLD) are two major epidemics affecting millions of people worldwide. As people with HIV (PWH) age, there is an increased prevalence of metabolic comorbidities, along with unique HIV factors, such as HIV chronic inflammation and life-long exposure to antiretroviral therapy, which leads to a high prevalence of NAFLD. Among people with HIV, nutrition has a key impact in both NAFLD onset and regression.
- HIV
- fatty liver
- MAFLD
- NAFLD therapy
- nutrients
- lifestyle
1. Nutrients in NAFLD
1.1. Macronutrients
Macronutrients have an impact on the multifactorial pathogenesis of NAFLD, being able to alter hepatic fat storage and liver inflammation [23][1]. In this section, we will discuss the roles of carbohydrates, lipids, proteins, alcohol, and coffee in NAFLD will be discussed.
Carbohydrates
Overfeeding with refined carbohydrates leads to an intrahepatic triglycerides accumulation, supporting the role of excessive carbohydrates consumption in hepatic de novo lipogenesis [24][2]. Special attention should be paid to soft drinks, which are beverages made with great amounts of high fructose (corn syrup) or sucrose, having been shown to be a risk factor for NAFLD independently of metabolic syndrome [25,26][3][4]. In contrast, the consumption of whole grains, oatmeal, and quinoa, which are very rich in vitamins, minerals, and fibers, shows a strong inverse correlation with NAFLD [27,28,29][5][6][7] and improves insulin sensitivity, with proven benefits for metabolic disease [30,31][8][9].
Lipids
As for carbohydrates, the type of fat being ingested has a great impact on the NAFLD pathophysiology, more so than the total amount consumed [32][10]. On the one hand, the dietary abuse of saturated long-chain fatty acids (FAs), which are commonly found in animal products, promotes hepatic oxidative stress and the impairment of insulin signaling, leading to fatty liver, insulin resistance, and NASH [23][1]. On the other hand, plant-based food, especially nuts, olive oil, and avocados, are good sources of dietary monounsaturated FAs and polyunsaturated FAs, which are both associated with the amelioration of hepatic steatosis and liver inflammation [33,34][11][12]. In addition, among polyunsaturated FAs, there is evidence of a more harmful effect of n-6 FA (linoleic acid) than n-3 FA (alpha-linolenic acid) on the accumulation of intrahepatic triglycerides, with a higher intake of n-6 FAs and an increased n-6/n-3 ratio in NASH patients compared to controls [35][13].
Proteins
Finally, there is conflicting evidence regarding the association between protein consumption and NAFLD. Although a high protein intake seems to reduce intrahepatic fat, it may also negatively impact insulin sensitivity [23][1]. Again, the source of the protein is critical. In fact, a moderately high protein intake from white meats and legumes seems to be beneficial, lowering the hepatic fat content and markers of liver damage [36][14]. Conversely, eating a large amount of red and processed meat is associated with NAFLD and insulin resistance, independently of saturated fat intake [37,38][15][16].
Alcohol
Another macronutrient relevant to NAFLD is alcohol. By definition, the alcohol intake in NAFLD is either absent or low to mild (less than 20 g/day for women and less than 30 g/day for men) [10][17], but its effects on this population are still debated. Among NAFLD patients, while some old retrospective studies pointing to a favorable association between moderate alcohol use and less severe histological liver damage [39[18][19],40], more recent research has shown that even mild drinking might lead to liver fibrosis progression [41,42][20][21]. Furthermore, in a prospective cohort of NASH patients, alcohol consumption was identified as the major risk factor for HCC [43][22]. Evidence from longitudinal cohorts is accumulating, with a recent systematic review concluding that, in patients with NAFLD, any amount of alcohol intake, even at low concentrations, seems to be harmful to the liver’s health [44][23]. As for cardiovascular outcomes, limited data are available about the effects of small alcohol consumption on the cardiovascular risk in NAFLD. In fact, one study has shown a protective effect of moderate alcohol intake on cardiovascular outcomes [45][24], while others have found no difference [41,46][20][25]. Finally, aligned with the latest evidence, the most recent American Association for the Study of Liver Diseases (AASLD) guidance states that alcohol abstinence may lower the risk of fibrosis progression and hepatic and extrahepatic cancers in patients with NAFLD [10][17].
Coffee
Coffee is the second most consumed beverage in the world after water [47][26]. Coffee contains several compounds with known antioxidant effects, such as caffeine, chlorogenic acid, cafestol, kahweol, trigenolline, and tocopherols. These antioxidants are known to improve a number of liver conditions, ranging from fibrosis to cirrhosis and HCC [48][27]. In the setting of NAFLD, a recent meta-analysis of five cross-sectional studies showed a positive correlation between a high coffee consumption and a lower liver fibrosis, although no clear agreement was reached on the required dose [49][28]. However, not all forms and methods of coffee preparation show the same beneficial effects on NAFLD. Anty et al. reported that regular filtered coffee intake was associated with a lower liver fibrosis, while espresso was not [50][29]. In fact, antioxidant compounds seem to be better preserved in filtered coffee than in espresso [51][30]. In addition, espresso, as well as Turkish or Scandinavian boiled coffee, are often consumed with refined sugar, which is harmful for NAFLD, as it is highly rich in fructose [49][28]. Similarly, consuming a latte or cappuccino, in which the coffee is added to milk, which is rich in saturated fat, can also be detrimental [52][31]. Finally, in a recent RCT evaluating the administration of coffee components to treat NAFLD in diabetic patients, a supplementation with caffeine and/or chlorogenic acid did not improve hepatic steatosis or liver stiffness [53][32].
1.2. Micronutrients
In addition to macronutrients, micronutrients also play a role in the complex pathogenesis of fatty liver; thus, expert opinions recommend the consumption of micronutrients with proven antioxidant and anti-inflammatory effects to prevent and treat NAFLD [54][33].
Vitamin E
Vitamin E, which similarly to polyunsaturated FAs can be found in olive oil, nuts, and green vegetables, is a lipophilic antioxidant engaged in cellular signaling and gene expression regulation, showing anti-inflammatory and anti-apoptotic properties [55][34]. In total, two RCTs, the PIVENS trial in adults with NASH [56][35] and the PIVOT trial in the pediatric population [57][36], showed that a vitamin E supplementation resulted in a significant resolution of NASH compared to a placebo. Although these trials were not able to demonstrate that a vitamin E supplementation reduces liver fibrosis, a recent retrospective study on 236 individuals with severe liver fibrosis and NASH found a positive correlation between vitamin E consumption and both a longer transplant-free survival and reduced rates of hepatic decompensation [58][37]. In line with these promising results, the administration of vitamin E is acknowledged in both the AASLD and the European Association for the Study of the Liver guidelines as a short-term therapeutic option for non-diabetic NASH patients with liver fibrosis or with a high necroinflammation and a risk of fast histologic progression [10,22][17][38].
Vitamin D
Vitamin D may play a role in NAFLD development, as its deficiency is able to activate Toll-like receptors, induce oxidative stress, and finally, increase liver inflammation [59][39]. In mouse models, low serum vitamin D promotes the progression of simple steatosis to NASH [60][40], while vitamin D receptor ligands prevent hepatic stellate cells activation and liver fibrosis [61][41]. Consistently, human studies have shown that having decreased serum vitamin D concentrations is common among NAFLD patients [62][42], with a recent meta-analysis reporting an inverse correlation between vitamin D serum levels and an increased risk of NAFLD [63][43]. While several preclinical studies have shown a beneficial role for vitamin D in fatty liver [64][44], so far, interventional human studies have failed to demonstrate that a vitamin D administration improves the histologic severity of NAFLD [65][45], although it is significantly associated with a reduced fasting glucose and insulin resistance [66][46]. This may be explained by the limited number of trials examining the role of vitamin D replacement in NAFLD and by the variability of the levels and methodologies used to detect serum vitamin D levels [67][47].
Polyphenols
Polyphenols, commonly found in fruits, vegetables, olive oil, coffee, red wine, and dark chocolate, might be involved in the pathophysiology of NAFLD by preventing oxidative stress and inflammation, promoting FA beta-oxidation, and modulating insulin resistance [68][48]. Several clinical trials have revealed intriguing early findings on the safety and efficacy of polyphenols administration for the treatment of NAFLD. Specifically, a supplementation with curcumin [69[49][50],70], Silymarin [71][51], or hesperidin [72][52] seems to improve the different features of NAFLD with a good safety profile, whereas the available evidence on resveratrol is conflicting [73][53]. However, despite the encouraging preliminary data, further well-designed RCTs are warranted to address the use of polyphenols in NAFLD management.
Cannabinoid
Another natural plant-based compound whose role is being studied in chronic liver diseases is cannabinoid. Evidence from pre-clinical models shows that a cannabinoid receptors 1 overexpression leads to de novo hepatic lipogenesis, along with an intrahepatic monounsaturated FAs accumulation. Oppositely, a cannabinoid receptors 1 blockage inhibits insulin resistance and hepatic steatosis [74][54]. Similarly, cannabinoid receptors 2 are known to contribute both directly—by inducing liver inflammation—and indirectly—by increasing the hepatic cannabinoid receptors 1 expression—to NAFLD, NASH, and liver fibrosis [74][54]. Aligned with these findings, human studies have confirmed an inverse association of cannabis consumption with NAFLD [75[55][56],76], as well as with its metabolic risk factors, namely obesity [77][57], diabetes [78][58], and metabolic syndrome [79][59]. However, although several preclinical studies have suggested cannabinoids as a potential treatment for NAFLD, so far, no RCTs have been able to demonstrate their effective role in reducing hepatic steatosis or fibrosis [80][60].
2. Nutrients in NAFLD in People with HIV
2.1. Macronutrients
Limited data are available on the role of nutrients in the setting of NAFLD in PWH, with no studies specifically addressing the roles of carbohydrates and proteins. Studies evaluating the impact of lipids, alcohol, and coffee on hepatic steatosis and/or liver fibrosis in PWH are shown in Table 1.
Table 1.
Studies evaluating the role of macronutrients in hepatic steatosis and/or liver fibrosis in people with HIV.
| Author, Year, Country | Study Design | Nutrients | Study Cohort | Patients’ Characteristics (Sex, Age, BMI) | HIV Features (Duration of HIV Infection, cART, Viral Suppression) | Diagnostic Method for NAFLD/ NASH/Liver Fibrosis |
Main Findings |
|---|---|---|---|---|---|---|---|
| Arendt et al., 2011, Canada [81][61] | Cross-sectional | Fatty Acids | 48 participants:
|
88][68]. Finally, in a longitudinal observational study on 1019 subjects from the same cohort, Yaya et al. not only confirmed a strong inverse association between a high coffee consumption (i.e., ≥ three cups/day) and advanced liver fibrosis, but also showed a role for coffee in mitigating alcohol’s negative effect on liver damage [89][69]. Indeed, a higher coffee intake was associated with a lower risk of liver fibrosis, even in HIV/HCV-coinfected patients with a high-risk alcohol consumption (i.e., >four alcoholic units/day for men and >three alcoholic units/day for women). Although no data regarding hepatic steatosis were available, patients with a high coffee intake and low-risk alcohol consumption (i.e., ≤four alcoholic units/day for men and ≤three alcoholic units/day for women) had a significantly lower prevalence of advanced liver fibrosis (FIB-4 ≥ 3.25) compared to subjects with a low coffee intake and low-risk alcohol consumption (11.4% vs. 77.3% respectively, p < 0.001) [89][69], possibly suggesting a beneficial role of drinking coffee in NAFLD in PWH. However, since all these studies addressed a population of HIV/HCV coinfected subjects, there is still a need for evidence specifically assessing the link between coffee and NAFLD in HIV monoinfection. Figure 1 shows the role of nutrients in NAFLD in PWH.
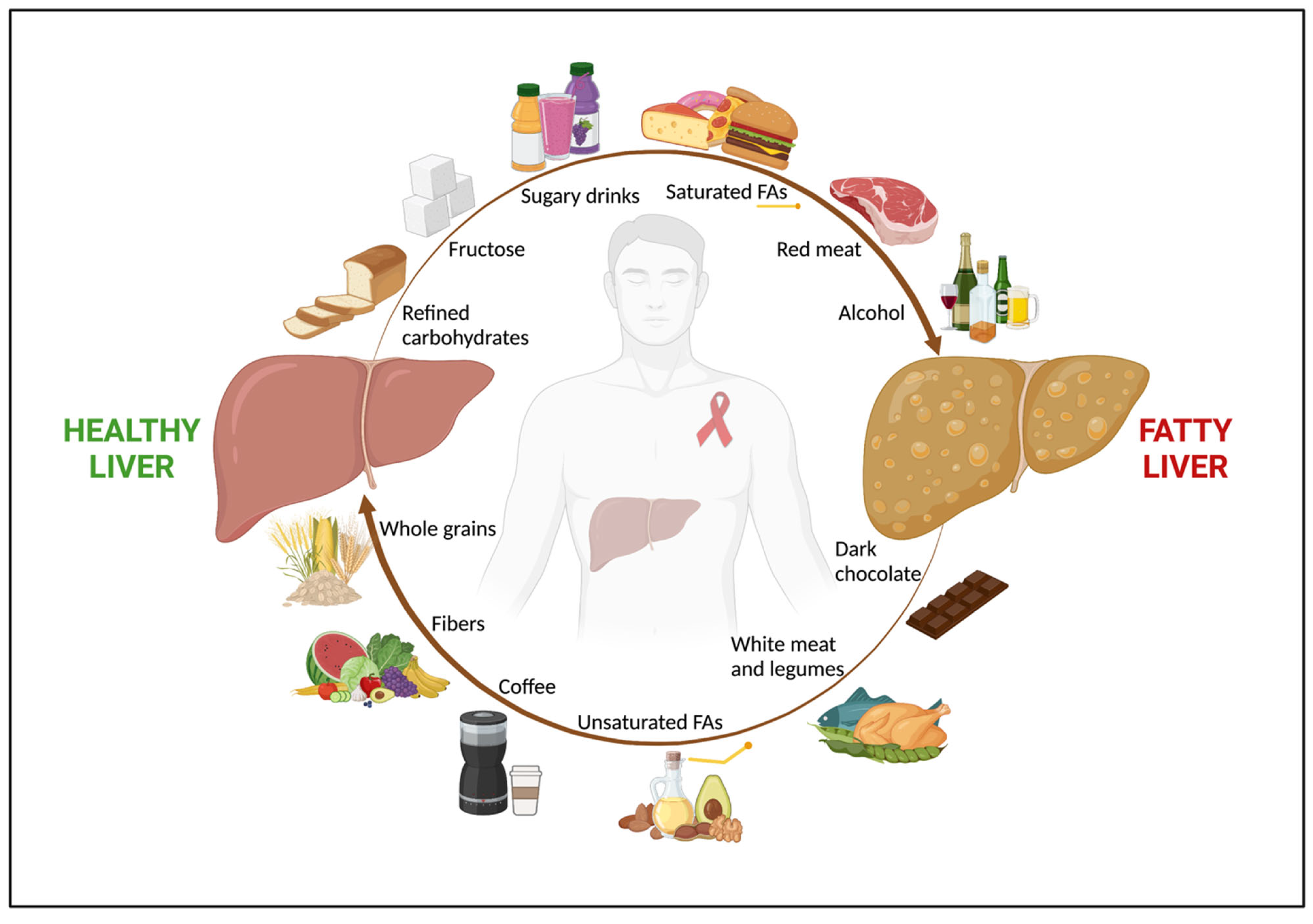
Figure 1. The role of nutrients in NAFLD in people with HIV. Among people with HIV, nutrition has a key impact in both NAFLD onset and regression. An unhealthy diet, rich in refined carbohydrates, fructose and sugar-added beverages, saturated FAs, and red and processed meat, along with alcohol abuse, are associated with fatty liver. Conversely, a healthy diet, with high intake of fruits, vegetables, whole grains, which are good sources of fibers, unsaturated FAs, white meat, dark chocolate, and coffee shows a beneficial effect on NAFLD. Abbreviations: NAFLD; non-alcoholic fatty liver disease; and FAs, fatty acids.
2.2. Micronutrients
As for the uninfected population, the roles of several micronutrients have been investigated among PWH with NAFLD. Studies addressing the role of micronutrients in hepatic steatosis and/or liver fibrosis in PWH are shown in Table 2.
Table 2.
Studies evaluating the role of micronutrients in hepatic steatosis and/or liver fibrosis in people with HIV.
| Author, Year, Country | Study Design | Nutrients of Interest | Study Cohort | Patients’ Characteristics (Sex, Age, BMI) | HIV Features (Duration of HIV Infection, cART, Viral Suppression) |
Diagnostic Method for NAFLD/ NASH/Liver Fibrosis |
Main Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sebastiani et al., 2020, Canada [105][83] | Single-center, open-label, single-arm, clinical trial, FU 4 years | Vitamin E | 27 PWH with NASH |
|
HIV/NAFLD: 95% on cART, | HS: liver biopsy | M 81%, age 54 (51–59) years, BMI 28 (25–32) kg/m2 | Duration HIV 23 (15–29) years, 100% on cART, 100% with viral suppression |
|
||||||||||
|
|
|
Martínez-Sanz et al., 2022, Spain [82][62] | Cross-sectional | Fatty Acids | 53 participants:
| |||||||||||||
| Guzman-Fulgencio et al., 2013, Spain [106][ |
|
84] |
|
Cross-sectional | Vitamin D |
|
HIV/NAFLD: 100% on cART, 100% with viral suppression | HS: Abdominal Ultrasound | 174 HIV/HCV-coinfected patients |
|
M 75%, age 40.8 (37.3; 44.6) years, | 86% on cART, 71% with viral suppression | Liver fibrosis: liver biopsy |
|
|
||||
|
| De Almeida et al., 2021, Brazil [83][63] | Cross-sectional | Fatty Acids, Carbohydrates, Fibers | 451 PWH | M 40%, age 45 (IQR 36–53) years, BMI 25 (23–29) kg/m2 | Duration HIV 10 (5–17) years, 97% on cART, 84% with viral suppression |
| |||||||||||
| Terrier et al., 2011, France [107][85 | ] | Cross-sectional | Vitamin D | 189 HIV/HCV-coinfected patients | M 77%, Age 39.5 (±4.8) years, BMI 22.7 (±3.2) kg/m2 |
|
Duration HIV 12 (0.5–18.5) years, 82% on cART, 70% with <400 HIV-RNA copies |
|
Liver fibrosis: liver biopsy |
|
|
||||||||
| Kelly et al., 2017, Canada/U.S. [84][64] | |||||||||||||||||||
| Milazzo et al., 2011, Italy [108][86 | Prospective, median FU 10 years | Alcohol | 686 HIV/HCV-coinfected patients | ] | Cross-sectional | Vitamin D | 237 patients
| F 100%, age 39.7 (±6) years, BMI 26.1 (±6), kg/m2 | 29% on cART |
| Liver fibrosis: FIB-4 |
|
|||||||
| Kirkegaard-Klitbo et al., 2020, Danmark [85][65] | Cross-sectional | Alcohol | 453 PWH | M 86%, age 52.4 (46.8–61.0) years, BMI 24.7 (22.4–27.5) kg/m2 | Duration HIV 16 (8.3–23.1) years, 99% on cART, 97% with viral suppression | HS: computed tomography |
|
||||||||||||
| Carrieri et al., 2014, France [86][66] | Prospective, median FU 3.3 years | Coffee, chocolate | 990 HIV/HCV coinfected patients (ANRS CO13 HEPAVIH cohort) | M 70%, age 45 (42–48) years | 91% on cART, 71% with viral suppression | N/A |
|
||||||||||||
| Carrieri et al., 2017, France [87][67] | Prospective, median FU 5 years | Coffee | 1028 HIV/HCV coinfected patients (ANRS CO13 HEPAVIH cohort) | M 70%, age 49 (46–52) years | 94.5% on ART, 82.5% with viral suppression | Liver fibrosis: FIB-4 |
|
||||||||||||
| Carrieri et al., 2018, France [88][68] | Cross-sectional | Coffee | 918 HIV/HCV coinfected patients (ANRS CO13 HEPAVIH cohort) | N/A | N/A | HS: N/A Liver fibrosis: LSM by TE and FIB-4 |
|
||||||||||||
| Yaya et al., 2018, France [89][69] | Cross-sectional | Coffee | 1019 HIV/HCV coinfected patients (ANRS CO13 HEPAVIH cohort) | M 70%, age 47.8 (±6.4) years | 95% on cART | Liver fibrosis: FIB-4 |
|
Legend: Continuous variables are expressed as mean + standard deviation or median (interquartile range or range) and categorical variables are presented as percentages. Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; cART, combined antiretroviral therapy; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PWH, people with HIV; M, males; F, females; FAs, fatty acids; FU, follow-up; HS, hepatic steatosis; CAP, Controlled Attenuation Parameter; N/A, not assessed; LSM, liver stiffness measurement; TE, transient elastography; and FIB-4, Fibrosis-4.
Lipids
As for the uninfected population, fat consumption plays a role in NAFLD pathogenesis in PWH. An altered hepatic FA composition was associated with NAFLD in PWH in a Canadian study comparing 20 PWH with NAFLD to 21 NAFLD uninfected subjects and 7 healthy controls [81][61]. The PWH with NAFLD showed a higher hepatic n-6/n-3 ratio, with a lower n-3 erythrocytes polyunsaturated FAs concentration (which reflects a lower polyunsaturated FAs dietary intake), and lower levels of the indirect markers of enzymatic FA desaturation and elongation compared to the controls. Interestingly, the altered FA composition was more impaired in the PWH with NAFLD compared to the uninfected NAFLD population [81][61]. Based on these results, FA imbalances, which are known to be major drivers of NAFLD, seem even more prominent in NAFLD in PWH. Indeed, the association between lipid imbalances and HIV infection is well established, with a high prevalence of dyslipidemia in PWH [90][70]. This might be caused both by HIV chronic inflammation, since lipid alterations were already reported before the use of cART [91][71], and a lifelong exposure to cART, especially to old PIs boosted with ritonavir and certain NRTIs [92][72]. Another recent study evaluated the changes in the plasma FAs profiles between 31 HIV-positive and 22 HIV-negative subjects with NAFLD [82][62]. Although they didn’t report any significant differences in the overall FA compositions, the authors showed higher concentrations of α-Linolenic, trans-palmitoleic, and behenic acids, all of which have been previously associated with metabolic alterations, in the PWH compared to the uninfected population. Additionally, the PWH had a lower activity of the elongases ELOVL1 and ELOVL6 (enzymes regulating the elongation of FAs), which have previously been found to be reduced in other chronic inflammatory diseases such as psoriasis. Interestingly, despite showing a similar hepatic steatosis severity, the PWH were significantly younger, with a lower body mass index (BMI) and a lower prevalence of metabolic syndrome compared to the controls [82][62], suggesting that an FA imbalance might occur earlier and at a lower BMI in the context of NAFLD in PWH. Focusing on omega 3 supplementation, although no study has specifically addressed NAFLD in PWH, in a meta-analysis of nine RCTs performed on PWH with hypertriglyceridemia, a diet supplementation with polyunsaturated FAs significantly improved triglycerides and high-density lipoprotein cholesterol, with a favorable safety profile [95][73]. Additionally, an omega-3 administration seemed to be also able to decrease systemic HIV-related inflammation, as measured by serum C-reactive protein [96][74]. Therefore, a dietary supplementation with omega-3 polyunsaturated FAs is recommended for PWH with hypertriglyceridemia, although further studies are warranted to evaluate its impact on NAFLD.
Alcohol
Alcohol consumption is common among PWH and its abuse is known to be associated with unfavorable clinical outcomes [97][75]. In fact, in the setting of HIV, drinking alcohol may lead to a low medication adherence, as well as to poor HIV control, with decreased CD4+ cells and elevated HIV-RNA levels, regardless of cART use [98,99][76][77]. In addition, by suppressing the innate and acquired immune system and causing a disruption in the gut barrier, alcohol intake seems to increase the susceptibility to HIV infection and accelerate the disease’s progression [100,101][78][79]. As for the liver’s health, alcohol metabolism may potentiate the effects of HIV-induced hepatotoxicity, because alcohol and HIV infection act on common targets to cause liver damage [102][80]. Ganesan et al., in a preclinical study, demonstrated that ethanol metabolism promotes an accumulation of HIV components in hepatocytes, causing oxidative stress and apoptotic cell death [103][81]. The underlying mechanism is potentially related to acetaldehyde-induced lysosomes damage, which enhances this HIV-induced hepatotoxicity [104][82]. Of note is that the removal of these HIV-infected apoptotic bodies by nonparenchymal liver cells triggers their activation, contributing to liver disease progression [103][81]. OurThe literature search shows a lack of clinical studies evaluating the impact of light to moderate alcohol intake on the liver outcomes among PWH, especially in the setting of NAFLD. In a large prospective cohort of 686 HIV/HCV-coinfected women, with a median follow-up of 10 years, light (1–3 drinks/week) or moderate (4–7 drinks/week) alcohol consumption was not associated with liver fibrosis progression, whereas drinking more than 14 drinks per week showed increased rates of fibrosis progression [84][64]. Therefore, Kelly and colleagues concluded that, although heavy alcohol consumption should be strongly discouraged in this population, complete abstinence might not be necessary [84][64]. Interestingly, a Danish study comparing 453 PWH without viral hepatitis and an excessive alcohol consumption to 765 healthy controls, showed that, in the HIV population, a moderate alcohol consumption (defined as <14 alcoholic units per week for men and <7 alcoholic units per week for women) was associated with lower odds of moderate-to-severe hepatic steatosis, in a multivariate analysis adjusted for age, sex, ethnicity, BMI, and physical activity level [85][65]. In addition, although a weekly consumption of beer was associated with lower odds of moderate-to-severe hepatic steatosis, no association was found with wine, liquor, sugar-sweetened beverages, coffee, fast food, and type of meat products [85][65]. However, in this study, the prevalence of moderate-to-severe hepatic steatosis (assessed by computed tomography) was only 8.6%, which is very low compared to the previous evidence on NAFLD in PWH and may have led to an underestimation of the alcohol effect. Further studies assessing the role of mild alcohol intake in NAFLD in PWH are needed.
Coffee
The anti-inflammatory and hepato-protective properties of coffee have also been studied in the setting of HIV. Evidence on this topic comes from a national multicenter prospective cohort of PWH coinfected with HCV in France (ANRS CO13 HEPAVIH) [86][66]. Firstly, in a subset of 990 subjects, Carrieri et al. showed that a higher coffee intake (≥three cups/day) was associated with a lower risk of impaired transaminases, with similar results for chocolate consumption [86][66]. Notably, combining a high intake of the two macronutrients together reduced the risk of abnormal liver enzymes by about 40% [86][66]. In 1028 HIV/HCV-coinfected patients with a median follow-up of 5 years, the same authors observed that a baseline high daily coffee intake of three or more cups was significantly associated with a 50% reduced risk in all-cause mortality [87][67]. Interestingly, looking at the baseline characteristics of the study cohort, the patients with a high coffee consumption were also less likely to present an advanced liver fibrosis in their Fibrosis-4 score (FIB-4) than the subjects with a moderate (i.e., two cups/day) or low (i.e., one cup/day, occasional consumption, or no consumption) coffee intake (10.7% vs. 14.3% vs. 19.7% respectively, p = 0.002) [87][67]. However, in this cohort, the effect of HCV infection may have been greater than that of HIV, being that HCV-related mortality was the first cause of death (42.8%), followed by non-HIV and non-HCV-related cancer (11.7%), AIDS (10.8%), and cardiovascular disease (3.9%) [87][67]. In a recent editorial, the same team further investigated the impact of coffee intake on significant liver steatosis and fibrosis among the patients of the ANRS CO13 HEPAVIH cohort [88][68]. Despite the lack of an association with hepatic steatosis, coffee intake was confirmed to have a significant protective effect on liver fibrosis, with a dose–response relationship for both the FIB-4 and liver stiffness measurements taken by transient elastography [
| |||||||
| |||||||
| |||||||
|
|
Liver fibrosis: FIB-4, liver biopsy (20% of the cohort) |
|
||||
| Mandorfer et al., 2015, [109][87] | Cross-sectional | Vitamin D | 86 HIV/HCV-coinfected patients | M 71%, age 38.7 (+9.6) years, BMI 23.2 (+4) kg/m2 | 71% on cART | Liver fibrosis: liver biopsy | Vitamin D deficiency was independently associated with liver fibrosis progression and higher portal pressure |
| El-Maouche et al., 2013 U.S. [110][88] | Cross-sectional | Vitamin D | 116 HIV/HCV-coinfected patients | M 63%, age 49.9 (46.5–53.3) years | 64% on cART, 79% with <400 HIV-RNA copies | Liver fibrosis: liver biopsy |
|
| Milic et al., 2020, Italy [111][89] | Cross-sectional | Vitamin D | 707 PWH | M 76%, age 53.5 (±8.2) years, BMI 24.6 (±4.2) kg/m2 | 100% on cART, 99% with viral suppression |
|
Vitamin D deficiency was associated with NAFLD with liver fibrosis |
| Nordmann et al., 2018, France [112][90] | Cross-sectional | Cannabinoids | 838 HIV/HCV-coinfected patients (ANRS CO13 HEPAVIH cohort) | M 70%, age 44.9 (44.5–45.4) years | 92% on cART, 84% with viral suppression | HS: abdominal ultrasound | Daily cannabis use was independently associated with lower prevalence of HS |
| Barré et al., 2021, France [113][91] | Prospective, FU 5 years | Cannabinoids | 997 HIV/HCV-coinfected patients (ANRS CO13 HEPAVIH cohort) | N/A | N/A | HS: fatty liver index | Regular cannabis use was associated with a 55% lower risk of elevated fatty liver index |
| Fuster et al., 2021, Russia [114][92] | Cross-sectional | Cannabinoids | 248 PWH with high prevalence of alcohol abuse (93%) and HCV coinfection (88%) | M 73%, age 33 (30–37) years, BMI 22.5 (20.8–24.5) kg/m2 | N/A | Liver fibrosis: FIB-4, APRI, LSM by TE | Cannabis use was not associated with advanced liver fibrosis |
| Kelly et al., 2016, USA [115][93] | Prospective, FU 11 years | Cannabinoids | 575 HIV/HCV-coinfected patients | Entry visit: F 100%, age 40 (±6) years, BMI 26 (±6) kg/m2Last visit: F 100%, age 51 (±7) years, BMI 26 (±7) kg/m2 | Entry visit: 6% on cART, 7% with viral suppression Last visit: 63% on cART, 33% with viral suppression |
Liver fibrosis: FIB-4, APRI | Cannabis use was not associated with progression to significant liver fibrosis |
| Brunet et al., 2014, Canada [116][94] | Prospective, FU 2.7 years | Cannabinoids | 690 HIV/HCV-coinfected patients | M 73%, age 43.9 (38.4–49.2) years, BMI 24.0 (21.2–26.8) kg/m2 | Duration HIV 18.0 (10.4–24.5) years, 54% with viral suppression | Liver fibrosis and cirrhosis: APRI | Cannabis use was not associated with liver fibrosis progression or cirrhosis |
Legend: Continuous variables are expressed as mean + standard deviation or median (interquartile range or range) and categorical variables are presented as percentages. Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; cART, combined antiretroviral therapy; NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; FU, follow-up; PWH, people with HIV; M, males; CAP, Controlled Attenuation Parameter; HS, hepatic steatosis; ALT, alanine transaminase; FIB-4, Fibrosis-4; LSM, liver stiffness measurement; TE, transient elastography; and APRI, AST to Platelet Ratio Index.
Vitamin E
Vitamin E deficiency seems to be common among PWH. For instance, in a cohort of 107 PWH in Ghana, a high prevalence (82.5%) of serum vitamin E insufficiency was detected, with a low dietary intake of this vitamin and other antioxidant micronutrients [117][95]. In addition, whether high vitamin E levels reduce the progression of HIV infection to AIDS is still under debate. In a study of 311 HIV-positive men in Baltimore, patients in the highest quartile of serum vitamin E levels showed a decreased risk of developing AIDS compared to those in the lowest quartile [118][96]. Oppositely, Graham and colleagues reported that higher vitamin E levels pre-infection were associated with a higher mortality among 67 Kenyan women with HIV [119][97]. More research is required on the clinical impact of vitamin E on the immunologic features of HIV. Focusing on the liver’s health, vitamin E is recommended for NASH treatment in the general population [10,22][17][38]. One of us designed a clinical trial to specifically investigate the effect of vitamin E supplementation on NASH in PWH [105][83]. This Canadian open-label phase 4 single-arm trial included 27 HIV mono-infected patients with non-invasive diagnoses of NASH, defined by a controlled attenuation parameter (CAP) of at least 248 dB/m and a serum cytokeratin 18 (marker of hepatocyte apoptosis) greater than 130.5 U/l. The daily administration of vitamin E 800 IU over 24 weeks led to a decrease in alanine transaminase levels and improvements in hepatic steatosis and hepatocyte apoptosis [105][83]. In addition, no major side effects were registered, confirming a good safety profile [105][83]. However, consistent with the findings for the general NAFLD population [56[35][36],57], no improvement in liver fibrosis was reported, possibly also due to the relatively short study duration [105][83]. Therefore, vitamin E may serve as a “bridge therapy” while awaiting novel drugs that will comprehensively treat all the features of NASH [120][98].
Vitamin D
Vitamin D seems to also play a role in NAFLD in PWH. Importantly, serum vitamin D deficiency is common among PWH, with a higher prevalence than that in the general population [121,122][99][100]. In fact, other than the traditional risk factors, such as a lack of exposure to UVB radiation, age, and a darker skin pigmentation, HIV chronic infection and the lifelong use of cART seem to exacerbate vitamin D deficiency in the setting of HIV [122][100]. Screening PWH for vitamin D insufficiency is clinically relevant due to the higher prevalence of osteopenia/osteoporosis and fractures in PWH and cART-treated individuals compared to controls [123][101]. Focusing on liver outcomes, in the setting of HIV/HCV coinfection, several studies have shown an association between vitamin D deficiency and increased biopsy-proven liver fibrosis [106,107,108][84][85][86]. Similarly, in a study evaluating 86 people with HIV/HCV coinfection, Mandorfer et al. reported that a vitamin D deficiency and low CD4+ nadir were independently associated with a higher portal pressure (assessed by a hepatic venous pressure gradient) and fibrosis progression rate (computed by dividing the METAVIR fibrosis score at liver biopsy by the duration of HCV infection expressed in years) [109][87]. Moreover, in a cohort of 139 PWH with advanced liver disease, who were candidates for liver transplantation, Branch et al. found a very high prevalence of vitamin D deficiency (90%), which was also independently associated with cirrhosis [124][102]. Conversely, El-Maouche and colleagues, in a cohort of 116 HIV/HCV-coinfected patients, although vitamin D deficiency was highly prevalent, there was no reported association between low vitamin D levels and liver fibrosis severity (by liver biopsy) or low bone mineral density (by dual-energy X-ray absorptiometry) [110][88].
Cannabinoids
In HIV infection, preliminary evidence has shown that cannabinoid intake may have a beneficial impact on its clinical outcomes. In fact, cannabis usage seems to be associated with lower levels of T-cell activation, inflammatory monocytes, and pro-inflammatory cytokine secretion, all of which correlate with both HIV progression and its comorbidities [127][103]. A few studies have investigated the role of cannabinoids in the liver’s health among PWH, with mixed results. The investigators of the ANRS CO13 HEPAVIH cohort demonstrated that, in the setting of HIV/HCV coinfection, daily cannabis use was negatively associated with being overweight [128][104], insulin resistance [129][105], and fatty liver, diagnosed by abdominal ultrasound [112][90] or the fatty liver index [113][91]. Oppositely, in 248 Russian PWH with heavy alcohol consumption, who were mostly male (72.6%), young (median age of 33.9 years), and coinfected with HCV (87.9%), Fuster et al. did not report any association between cannabis use and advanced liver fibrosis (detected by FIB-4, aminotransferase-to-platelet ratio (APRI), or transient elastography) [114][92]. Along the same lines, in a large longitudinal cohort of 575 HIV/HCV-coinfected women who were followed for a median of 11 years, marijuana usage was not associated with a progression to significant liver fibrosis (diagnosed by FIB-4 or APRI) [115][93]. Similar results were also shown by Brunet and colleagues, who detected no evidence that marijuana smoking accelerated significant liver fibrosis progression (detected by APRI) or cirrhosis in a prospective cohort of 690 Canadian HIV/HCV-coinfected individuals [116][94]. However, a longer follow-up than that of this study (2.7 years, interquartile range 0.8–3.8) may be needed to detect a significant rate of liver fibrosis and cirrhosis progression [116][94]. Of note is that recreational and medical cannabis use is also associated with negative cardiovascular, respiratory, cognitive, and psychological effects, although a definitive causal relationship between cannabis use and these adverse effects is lacking [130][106]. Therefore, these risks should be carefully considered before suggesting the use of cannabinoids. In addition, despite preclinical studies that have focused on isolated phytocannabinoids as therapeutic options for NAFLD showing promising results, preliminary human trials have failed to confirm them [74][54]. We believe that further evidence on safety and tolerability is needed before conducting large-scale efficacy human trials. In fact, as recently reported by one of us in a randomized interventional pilot study investigating the safety and tolerability of oral cannabinoids in 10 PWH, 2 patients developed elevated transaminases when receiving high doses of cannabinoids (800 mg per day) [131][107]. Therefore, it is still unclear what the optimal dose of cannabinoids is to determine their anti-inflammatory properties without causing hepatotoxicity.
3. Food Insecurity
When discussing nutrition and chronic diseases, in addition to the type and amount of nutrients consumed, it is relevant to mention food insecurity. Food insecurity is defined as the inability to acquire or consume an adequate diet quality or a sufficient quantity of food in socially acceptable ways, or the uncertainty that one will be able to do so [132][108]. Notably, it is not only a concern for low-income countries, but also for high-income ones, as it affects more than 12% of households in the United States [133][109]. Indeed, all these poverty-related unhealthy habits can lead to an increase in metabolic syndrome, obesity, hypertension, and diabetes [136][110]. The liver’s health is also dramatically affected by health disparities, with social determinants being crucial to the development and management of chronic liver diseases [137][111]. Focusing on hepatic steatosis, Golovaty et al., in a large cohort of 2627 adults in the United States, reported that food-insecure subjects were more likely to have NAFLD (assessed by the fatty liver index) and advanced liver fibrosis (assessed by NAFLD fibrosis score) compared to food-secure adults [138][112]. Of note is that in another, even larger population-based study, including 4816 subjects with NAFLD (diagnosed by fatty liver index) and 1654 patients with advanced liver fibrosis (diagnosed by NAFLD fibrosis score, APRI, or FIB-4), food insecurity was an independent predictor of a higher mortality rate for both groups, also being significantly associated with a greater outpatient health care utilization among the NAFLD subjects [139][113]. In addition, PWH seem to be even more vulnerable to food insecurity than the general population, being that HIV infection is disproportionately affected by economic disadvantages and social stigmas [140][114]. Consistent with the findings for the general population, Muhammad et al. reported that food-insecure PWH had a poor diet quality, with a low dietary intake of fiber, vitamin E, folate, magnesium, and copper [141][115]. Among 603 subjects of the Miami Adult Studies on HIV cohort (43.6% HIV-positive), who were screened for NAFLD and liver fibrosis with magnetic resonance imaging, Tamargo and colleagues showed that food insecurity was linked to increased odds for NAFLD at a higher BMI, and that it was independently associated with a higher risk of liver fibrosis [142][116]. Finally, wthe researchers would like to emphasize that the health promotion of PWH with NAFLD cannot be limited to nutritional advice alone. In fact, it is a matter of human rights to ensure that all patients, even the most underprivileged and those with the lowest incomes, have equal access to quality food. It is critical that health policies take action to remove the socioeconomic barriers that force these vulnerable patients into food insecurity, exposing them to an increased risk of serious illness and death.
References
- Chakravarthy, M.V.; Waddell, T.; Banerjee, R.; Guess, N. Nutrition and Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. North Am. 2020, 49, 63–94.
- Sevastianova, K.; Santos, A.; Kotronen, A.; Hakkarainen, A.; Makkonen, J.; Silander, K.; Peltonen, M.; Romeo, S.; Lundbom, J.; Lundbom, N.; et al. Effect of Short-Term Carbohydrate Overfeeding and Long-Term Weight Loss on Liver Fat in Overweight Humans. Am. J. Clin. Nutr. 2012, 96, 727–734.
- Nseir, W.; Nassar, F.; Assy, N. Soft Drinks Consumption and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2010, 16, 2579–2588.
- Abid, A.; Taha, O.; Nseir, W.; Farah, R.; Grosovski, M.; Assy, N. Soft Drink Consumption Is Associated with Fatty Liver Disease Independent of Metabolic Syndrome. J. Hepatol. 2009, 51, 918–924.
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association Between Dietary Fiber Intake and Non-Alcoholic Fatty Liver Disease in Adults. Front. Nutr. 2020, 7, 593735.
- Zolfaghari, H.; Askari, G.; Siassi, F.; Feizi, A.; Sotoudeh, G. Intake of Nutrients, Fiber, and Sugar in Patients with Nonalcoholic Fatty Liver Disease in Comparison to Healthy Individuals. Int. J. Prev. Med. 2016, 7, 98.
- Krawczyk, M.; Maciejewska, D.; Ryterska, K.; Czerwińka-Rogowska, M.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Milkiewicz, P.; Raszeja-Wyszomirska, J.; Stachowska, E. Gut Permeability Might Be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients 2018, 10, 1793.
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209.
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obes. Control Ther. 2017, 4, 7058–7069.
- Parry, S.A.; Hodson, L. Influence of Dietary Macronutrients on Liver Fat Accumulation and Metabolism. J. Investig. Med. 2017, 65, 1102–1115.
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.K.; Basu, A.; Kremers, W.K.; et al. Randomized Controlled Trial of a MUFA or Fiber-Rich Diet on Hepatic Fat in Prediabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1765–1774.
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged N-3 Polyunsaturated Fatty Acid Supplementation Ameliorates Hepatic Steatosis in Patients with Non-Alcoholic Fatty Liver Disease: A Pilot Study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151.
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How Different Is the Dietary Pattern in Non-Alcoholic Steatohepatitis Patients? Clin. Nutr. 2006, 25, 816–823.
- Tricò, D.; Biancalana, E.; Solini, A. Protein and Amino Acids in Nonalcoholic Fatty Liver Disease. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 96–101.
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High Red and Processed Meat Consumption Is Associated with Non-Alcoholic Fatty Liver Disease and Insulin Resistance. J. Hepatol. 2018, 68, 1239–1246.
- Hashemian, M.; Merat, S.; Poustchi, H.; Jafari, E.; Radmard, A.-R.; Kamangar, F.; Freedman, N.; Hekmatdoost, A.; Sheikh, M.; Boffetta, P.; et al. Red Meat Consumption and Risk of Nonalcoholic Fatty Liver Disease in a Population with Low Meat Consumption: The Golestan Cohort Study. Am. J. Gastroenterol. 2021, 116, 1667–1675.
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023.
- Dunn, W.; Sanyal, A.J.; Brunt, E.M.; Unalp-Arida, A.; Donohue, M.; McCullough, A.J.; Schwimmer, J.B. Modest Alcohol Consumption Is Associated with Decreased Prevalence of Steatohepatitis in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). J. Hepatol. 2012, 57, 384–391.
- Kwon, H.K.; Greenson, J.K.; Conjeevaram, H.S. Effect of Lifetime Alcohol Consumption on the Histological Severity of Non-Alcoholic Fatty Liver Disease. Liver Int. 2014, 34, 129–135.
- Kashiwagi, K.; Yamaguchi, A.; Shiba, S.; Taniki, N.; Inoue, N.; Takaishi, H.; Iwao, Y.; Kanai, T. Moderate Alcohol Consumption Is Not Associated with Subclinical Cardiovascular Damage but with Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease. Alcohol 2020, 89, 1–7.
- Blomdahl, J.; Nasr, P.; Ekstedt, M.; Kechagias, S. Moderate Alcohol Consumption Is Associated with Advanced Fibrosis in Non-Alcoholic Fatty Liver Disease and Shows a Synergistic Effect with Type 2 Diabetes Mellitus. Metabolism 2021, 115, 154439.
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The Incidence and Risk Factors of Hepatocellular Carcinoma in Patients with Nonalcoholic Steatohepatitis. Hepatology 2010, 51, 1972–1978.
- Jarvis, H.; O’Keefe, H.; Craig, D.; Stow, D.; Hanratty, B.; Anstee, Q.M. Does Moderate Alcohol Consumption Accelerate the Progression of Liver Disease in NAFLD? A Systematic Review and Narrative Synthesis. BMJ Open 2022, 12, e049767.
- Sinn, D.H.; Gwak, G.-Y.; Cho, J.; Son, H.J.; Paik, Y.-H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Modest Alcohol Consumption and Carotid Plaques or Carotid Artery Stenosis in Men with Non-Alcoholic Fatty Liver Disease. Atherosclerosis 2014, 234, 270–275.
- VanWagner, L.B.; Ning, H.; Allen, N.B.; Ajmera, V.; Lewis, C.E.; Carr, J.J.; Lloyd-Jones, D.M.; Terrault, N.A.; Siddique, J. Alcohol Use and Cardiovascular Disease Risk in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 153, 1260–1272.e3.
- Butt, M.S.; Sultan, M.T. Coffee and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373.
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46.
- Sewter, R.; Heaney, S.; Patterson, A. Coffee Consumption and the Progression of NAFLD: A Systematic Review. Nutrients 2021, 13, 2381.
- Anty, R.; Marjoux, S.; Iannelli, A.; Patouraux, S.; Schneck, A.-S.; Bonnafous, S.; Gire, C.; Amzolini, A.; Ben-Amor, I.; Saint-Paul, M.-C.; et al. Regular Coffee but Not Espresso Drinking Is Protective against Fibrosis in a Cohort Mainly Composed of Morbidly Obese European Women with NAFLD Undergoing Bariatric Surgery. J. Hepatol. 2012, 57, 1090–1096.
- Saab, S.; Mallam, D.; Cox II, G.A.; Tong, M.J. Impact of Coffee on Liver Diseases: A Systematic Review. Liver Int. 2014, 34, 495–504.
- Dranoff, J.A. Coffee Consumption and Prevention of Cirrhosis: In Support of the Caffeine Hypothesis. Gene Expr. 2018, 18, 1–3.
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of Supplementation with Main Coffee Components Including Caffeine and/or Chlorogenic Acid on Hepatic, Metabolic, and Inflammatory Indices in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Nutr. J. 2021, 20, 35.
- Dongiovanni, P.; Lanti, C.; Riso, P.; Valenti, L. Nutritional Therapy for Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2016, 29, 1–11.
- Perumpail, B.; Li, A.; John, N.; Sallam, S.; Shah, N.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86.
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685.
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents. JAMA 2011, 305, 1659–1668.
- Vilar-Gomez, E.; Vuppalanchi, R.; Gawrieh, S.; Ghabril, M.; Saxena, R.; Cummings, O.W.; Chalasani, N. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients with Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology 2020, 71, 495–509.
- EASL–EASD–EASO. Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402.
- Kwok, R.M.; Torres, D.M.; Harrison, S.A. Vitamin D and Nonalcoholic Fatty Liver Disease (NAFLD): Is It More than Just an Association? Hepatology 2013, 58, 1166–1174.
- Kong, M.; Zhu, L.; Bai, L.; Zhang, X.; Chen, Y.; Liu, S.; Zheng, S.; Pandol, S.J.; Han, Y.-P.; Duan, Z. Vitamin D Deficiency Promotes Nonalcoholic Steatohepatitis through Impaired Enterohepatic Circulation in Animal Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G883–G893.
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A Vitamin D Receptor/SMAD Genomic Circuit Gates Hepatic Fibrotic Response. Cell 2013, 153, 601–613.
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-Analysis: Vitamin D and Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254.
- Liu, T.; Xu, L.; Chen, F.-H.; Zhou, Y.-B. Association of Serum Vitamin D Level and Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 140–147.
- Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10705.
- Jaruvongvanich, V.; Ahuja, W.; Sanguankeo, A.; Wijarnpreecha, K.; Upala, S. Vitamin D and Histologic Severity of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2017, 49, 618–622.
- Guo, X.; Wang, C.; Yang, T.; Li, S.; Li, K.; Li, D. Vitamin D and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 7389–7399.
- Eliades, M.; Spyrou, E. Vitamin D: A New Player in Non-Alcoholic Fatty Liver Disease? World J. Gastroenterol. 2015, 21, 1718–1727.
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494.
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-Alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-Controlled Trial. Phytother. Res. 2016, 30, 1540–1548.
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251.
- Zhong, S.; Fan, Y.; Yan, Q.; Fan, X.; Wu, B.; Han, Y.; Zhang, Y.; Chen, Y.; Zhang, H.; Niu, J. The Therapeutic Effect of Silymarin in the Treatment of Nonalcoholic Fatty Disease. Medicine 2017, 96, e9061.
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin Improves Hepatic Steatosis, Hepatic Enzymes, and Metabolic and Inflammatory Parameters in Patients with Nonalcoholic Fatty Liver Disease: A Randomized, Placebo-controlled, Double-blind Clinical Trial. Phytother. Res. 2019, 33, 2118–2125.
- Jakubczyk, K.; Skonieczna-Żydecka, K.; Kałduńska, J.; Stachowska, E.; Gutowska, I.; Janda, K. Effects of Resveratrol Supplementation in Patients with Non-Alcoholic Fatty Liver Disease—A Meta-Analysis. Nutrients 2020, 12, 2435.
- Mboumba Bouassa, R.-S.; Sebastiani, G.; Di Marzo, V.; Jenabian, M.-A.; Costiniuk, C.T. Cannabinoids and Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 9423.
- Adejumo, A.C.; Alliu, S.; Ajayi, T.O.; Adejumo, K.L.; Adegbala, O.M.; Onyeakusi, N.E.; Akinjero, A.M.; Durojaiye, M.; Bukong, T.N. Cannabis Use Is Associated with Reduced Prevalence of Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. PLoS ONE 2017, 12, e0176416.
- Kim, D.; Kim, W.; Kwak, M.-S.; Chung, G.E.; Yim, J.Y.; Ahmed, A. Inverse Association of Marijuana Use with Nonalcoholic Fatty Liver Disease among Adults in the United States. PLoS ONE 2017, 12, e0186702.
- Le Strat, Y.; Le Foll, B. Obesity and Cannabis Use: Results from 2 Representative National Surveys. Am. J. Epidemiol. 2011, 174, 929–933.
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased Prevalence of Diabetes in Marijuana Users: Cross-Sectional Data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012, 2, e000494.
- Vidot, D.C.; Prado, G.; Hlaing, W.M.; Florez, H.J.; Arheart, K.L.; Messiah, S.E. Metabolic Syndrome Among Marijuana Users in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am. J. Med. 2016, 129, 173–179.
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Cannabidiol Dosing in Clinical Populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900.
- Arendt, B.M.; Mohammed, S.S.; Ma, D.W.L.; Aghdassi, E.; Salit, I.E.; Wong, D.K.H.; Guindi, M.; Sherman, M.; Heathcote, E.J.; Allard, J.P. Non-Alcoholic Fatty Liver Disease in HIV Infection Associated with Altered Hepatic Fatty Acid Composition. Curr. HIV Res. 2011, 9, 128–135.
- Martínez-Sanz, J.; Calvo, M.V.; Serrano-Villar, S.; Montes, M.L.; Martín-Mateos, R.; Burgos-Santamaría, D.; Díaz-Álvarez, J.; Talavera-Rodríguez, A.; Rosas, M.; Moreno, S.; et al. Effects of HIV Infection in Plasma Free Fatty Acid Profiles among People with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 3842.
- De Almeida, C.F.; da Silva, P.S.; Cardoso, C.S.D.A.; Moreira, N.G.; Antunes, J.C.; de Andrade, M.M.; Silva, J.; Araujo, M.C.; Peres, W.A.F.; do Brasil, P.E.A.A.; et al. Relationship between Dietary Fatty Acid Intake with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in People with HIV. Nutrients 2021, 13, 3462.
- Kelly, E.M.; Dodge, J.L.; Bacchetti, P.; Sarkar, M.; French, A.L.; Tien, P.C.; Glesby, M.J.; Golub, E.T.; Augenbraun, M.; Plankey, M.; et al. Moderate Alcohol Use Is Not Associated with Fibrosis Progression in Human Immunodeficiency Virus/Hepatitis C Virus–Coinfected Women: A Prospective Cohort Study. Clin. Infect. Dis. 2017, 65, 2050–2056.
- Kirkegaard-Klitbo, D.M.; Fuchs, A.; Stender, S.; Sigvardsen, P.E.; Kühl, J.T.; Kofoed, K.F.; Køber, L.; Nordestgaard, B.G.; Bendtsen, F.; Mocroft, A.; et al. Prevalence and Risk Factors of Moderate-to-Severe Hepatic Steatosis in Human Immunodeficiency Virus Infection: The Copenhagen Co-Morbidity Liver Study. J. Infect. Dis. 2020, 222, 1353–1362.
- Carrieri, M.P.; Lions, C.; Sogni, P.; Winnock, M.; Roux, P.; Mora, M.; Bonnard, P.; Salmon, D.; Dabis, F.; Spire, B. Association between Elevated Coffee Consumption and Daily Chocolate Intake with Normal Liver Enzymes in HIV-HCV Infected Individuals: Results from the ANRS CO13 HEPAVIH Cohort Study. J. Hepatol. 2014, 60, 46–53.
- Carrieri, M.P.; Protopopescu, C.; Marcellin, F.; Rosellini, S.; Wittkop, L.; Esterle, L.; Zucman, D.; Raffi, F.; Rosenthal, E.; Poizot-Martin, I.; et al. Protective Effect of Coffee Consumption on All-Cause Mortality of French HIV-HCV Co-Infected Patients. J. Hepatol. 2017, 67, 1157–1167.
- Carrieri, M.P.; Protopopescu, C.; Marcellin, F.; Wittkop, L.; Lacombe, K.; Esterle, L.; Sogni, P.; Salmon-Ceron, D. The Impact of Coffee Consumption on Fibrosis and Steatosis in HIV-HCV Co-Infected Patients. J. Hepatol. 2018, 68, 845–847.
- Yaya, I.; Marcellin, F.; Costa, M.; Morlat, P.; Protopopescu, C.; Pialoux, G.; Santos, M.E.; Wittkop, L.; Esterle, L.; Gervais, A.; et al. Impact of Alcohol and Coffee Intake on the Risk of Advanced Liver Fibrosis: A Longitudinal Analysis in HIV-HCV Coinfected Patients (ANRS CO-13 HEPAVIH Cohort). Nutrients 2018, 10, 705.
- Sprinz, E.; Lazzaretti, R.K.; Kuhmmer, R.; Ribeiro, J.P. Dyslipidemia in HIV-Infected Individuals. Braz. J. Infect. Dis. 2010, 14, 575–588.
- Grunfeld, C.; Pang, M.; Doerrler, W.; Shigenaga, J.K.; Jensen, P.; Feingold, K.R. Lipids, Lipoproteins, Triglyceride Clearance, and Cytokines in Human Immunodeficiency Virus Infection and the Acquired Immunodeficiency Syndrome. J. Clin. Endocrinol. Metab 1992, 74, 1045–1052.
- Funderburg, N.T.; Mehta, N.N. Lipid Abnormalities and Inflammation in HIV Inflection. Curr. HIV/AIDS Rep. 2016, 13, 218–225.
- Fogacci, F.; Strocchi, E.; Veronesi, M.; Borghi, C.; Cicero, A.F.G. Effect of Omega-3 Polyunsaturated Fatty Acids Treatment on Lipid Pattern of HIV Patients: A Meta-Analysis of Randomized Clinical Trials. Mar. Drugs 2020, 18, 292.
- Morvaridzadeh, M.; Sepidarkish, M.; Yavari, M.; Tahvilian, N.; Heydarian, A.; Khazdouz, M.; Farsi, F.; Persad, E.; Heshmati, J. The Effects of Omega-3 Fatty Acid Supplementation on Inflammatory Factors in HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Cytokine 2020, 136, 155298.
- Seth, A.; Sherman, K.E. Fatty Liver Disease in Persons with HIV Infection. Top. Antivir. Med. 2019, 27, 75–82.
- Chaudhry, A.A.; Sulkowski, M.S.; Chander, G.; Moore, R.D. Hazardous Drinking Is Associated with an Elevated Aspartate Aminotransferase to Platelet Ratio Index in an Urban HIV-Infected Clinical Cohort. HIV Med. 2009, 10, 133–142.
- Baum, M.K.; Rafie, C.; Lai, S.; Sales, S.; Page, J.B.; Campa, A. Alcohol Use Accelerates HIV Disease Progression. AIDS Res. Hum. Retrovir. 2010, 26, 511–518.
- Hahn, J.A.; Samet, J.H. Alcohol and HIV Disease Progression: Weighing the Evidence. Curr. HIV/AIDS Rep. 2010, 7, 226–233.
- Balagopal, A.; Philp, F.H.; Astemborski, J.; Block, T.M.; Mehta, A.; Long, R.; Kirk, G.D.; Mehta, S.H.; Cox, A.L.; Thomas, D.L.; et al. Human Immunodeficiency Virus-Related Microbial Translocation and Progression of Hepatitis C. Gastroenterology 2008, 135, 226–233.
- Szabo, G. Mechanisms of Alcohol-Mediated Hepatotoxicity in Human-Immunodeficiency-Virus-Infected Patients. WJG 2011, 17, 2500.
- Ganesan, M.; New-Aaron, M.; Dagur, R.S.; Makarov, E.; Wang, W.; Kharbanda, K.K.; Kidambi, S.; Poluektova, L.Y.; Osna, N.A. Alcohol Metabolism Potentiates HIV-Induced Hepatotoxicity: Contribution to End-Stage Liver Disease. Biomolecules 2019, 9, 851.
- New-Aaron, M.; Thomes, P.G.; Ganesan, M.; Dagur, R.S.; Donohue, T.M.; Kusum, K.K.; Poluektova, L.Y.; Osna, N.A. Alcohol-Induced Lysosomal Damage and Suppression of Lysosome Biogenesis Contribute to Hepatotoxicity in HIV-Exposed Liver Cells. Biomolecules 2021, 11, 1497.
- Sebastiani, G.; Saeed, S.; Lebouche, B.; de Pokomandy, A.; Szabo, J.; Haraoui, L.-P.; Routy, J.-P.; Wong, P.; Deschenes, M.; Ghali, P.; et al. Vitamin E Is an Effective Treatment for Nonalcoholic Steatohepatitis in HIV Mono-Infected Patients. AIDS 2020, 34, 237–244.
- Guzmán-Fulgencio, M.; García-Álvarez, M.; Berenguer, J.; Jiménez-Sousa, M.Á.; Cosín, J.; Pineda-Tenor, D.; Carrero, A.; Aldámiz, T.; Álvarez, E.; López, J.C.; et al. Vitamin D Deficiency Is Associated with Severity of Liver Disease in HIV/HCV Coinfected Patients. J. Infect. 2014, 68, 176–184.
- Terrier, B.; Carrat, F.; Geri, G.; Pol, S.; Piroth, L.; Halfon, P.; Poynard, T.; Souberbielle, J.-C.; Cacoub, P. Low 25-OH Vitamin D Serum Levels Correlate with Severe Fibrosis in HIV-HCV Co-Infected Patients with Chronic Hepatitis. J. Hepatol. 2011, 55, 756–761.
- El-Maouche, D.; Mehta, S.H.; Sutcliffe, C.G.; Higgins, Y.; Torbenson, M.S.; Moore, R.D.; Thomas, D.L.; Sulkowski, M.S.; Brown, T.T. Vitamin D Deficiency and Its Relation to Bone Mineral Density and Liver Fibrosis in HIV–HCV Coinfection. Antivir. Ther. 2013, 18, 237–242.
- Milic, J.; Menozzi, V.; Schepis, F.; Malagoli, A.; Besutti, G.; Franconi, I.; Raimondi, A.; Carli, F.; Mussini, C.; Sebastiani, G.; et al. Liver Steatosis and Nonalcoholic Fatty Liver Disease with Fibrosis Are Predictors of Frailty in People Living with HIV. AIDS 2020, 34, 1915–1921.
- Nordmann, S.; Vilotitch, A.; Roux, P.; Esterle, L.; Spire, B.; Marcellin, F.; Salmon-Ceron, D.; Dabis, F.; Chas, J.; Rey, D.; et al. Daily Cannabis and Reduced Risk of Steatosis in Human Immunodeficiency Virus and Hepatitis C Virus-Co-Infected Patients (ANRS CO13-HEPAVIH). J. Viral Hepat. 2018, 25, 171–179.
- Barré, T.; Rojas Rojas, T.; Lacombe, K.; Protopopescu, C.; Poizot-Martin, I.; Nishimwe, M.L.; Zucman, D.; Esterle, L.; Billaud, E.; Aumaitre, H.; et al. Cannabis Use and Reduced Risk of Elevated Fatty Liver Index in HIV-HCV Co-Infected Patients: A Longitudinal Analysis (ANRS CO13 HEPAVIH). Expert Rev. Anti-Infect. Ther. 2021, 19, 1147–1156.
- Fuster, D.; So-Armah, K.; Cheng, D.M.; Coleman, S.M.; Gnatienko, N.; Lioznov, D.; Krupitsky, E.M.; Freiberg, M.S.; Samet, J.H. Lack of Association between Recent Cannabis Use and Advanced Liver Fibrosis among HIV-Positive Heavy Drinkers. Curr. HIV Res. 2021, 19, 324–331.
- Kelly, E.M.; Dodge, J.L.; Sarkar, M.; French, A.L.; Tien, P.C.; Glesby, M.J.; Golub, E.T.; Augenbraun, M.; Plankey, M.; Peters, M.G. Marijuana Use Is Not Associated with Progression to Advanced Liver Fibrosis in HIV/Hepatitis C Virus–Coinfected Women. Clin. Infect. Dis. 2016, 63, 512–518.
- Brunet, L.; Moodie, E.E.M.; Rollet, K.; Cooper, C.; Walmsley, S.; Potter, M.; Klein, M.B. Marijuana Smoking Does Not Accelerate Progression of Liver Disease in HIV–Hepatitis C Coinfection: A Longitudinal Cohort Analysis. Clin. Infect. Dis. 2013, 57, 663–670.
- Kpewou, D.E.; Mensah, F.O.; Appiah, C.A.; Alidu, H.W.; Badii, V.S. Serum Vitamin E Deficiency among People Living with HIV and Undergoing Antiretroviral Therapy at Ho Teaching Hospital, Ghana. Heliyon 2021, 7, e07339.
- Tang, A.M.; Graham, N.M.; Semba, R.D.; Saah, A.J. Association between Serum Vitamin A and E Levels and HIV-1 Disease Progression. AIDS 1997, 11, 613–620.
- Graham, S.M.; Baeten, J.M.; Richardson, B.A.; Bankson, D.D.; Lavreys, L.; Ndinya-Achola, J.O.; Mandaliya, K.; Overbaugh, J.; McClelland, R.S. Higher Pre-Infection Vitamin E Levels Are Associated with Higher Mortality in HIV-1-Infected Kenyan Women: A Prospective Study. BMC Infect. Dis. 2007, 7, 63.
- Guaraldi, G.; Milic, J. Vitamin E as a ‘Bridge’ Therapy for Nonalcoholic Steatohepatits in HIV: What Is Waiting on the Other Side of the Bridge? AIDS 2020, 34, 317–319.
- Dao, C.N.; Patel, P.; Overton, E.T.; Rhame, F.; Pals, S.L.; Johnson, C.; Bush, T.; Brooks, J.T. The Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN) Investigators Low Vitamin D among HIV-Infected Adults: Prevalence of and Risk Factors for Low Vitamin D Levels in a Cohort of HIV-Infected Adults and Comparison to Prevalence among Adults in the US General Population. Clin. Infect. Dis. 2011, 52, 396–405.
- Chokuda, E.; Reynolds, C.; Das, S. Association of Low Vitamin D with Complications of HIV and AIDS: A Literature Review. Infect. Disord. Drug Targets 2020, 20, 122–142.
- Goh, S.S.L.; Lai, P.S.M.; Tan, A.T.B.; Ponnampalavanar, S. Reduced Bone Mineral Density in Human Immunodeficiency Virus-Infected Individuals: A Meta-Analysis of Its Prevalence and Risk Factors: Supplementary Presentation. Osteoporos. Int. 2018, 29, 1683.
- Milazzo, L.; Mazzali, C.; Bestetti, G.; Longhi, E.; Foschi, A.; Viola, A.; Vago, T.; Galli, M.; Parravicini, C.; Antinori, S. Liver-Related Factors Associated with Low Vitamin D Levels in HIV and HIV/HCV Coinfected Patients and Comparison to General Population. Curr. HIV Res. 2011, 9, 186–193.
- Mandorfer, M.; Payer, B.A.; Schwabl, P.; Steiner, S.; Ferlitsch, A.; Aichelburg, M.C.; Stättermayer, A.F.; Ferenci, P.; Obermayer-Pietsch, B.; Grabmeier-Pfistershammer, K.; et al. Revisiting Liver Disease Progression in HIV/HCV-Coinfected Patients: The Influence of Vitamin D, Insulin Resistance, Immune Status, IL28B and PNPLA3. Liver Int. 2015, 35, 876–885.
- Branch, A.D.; Barin, B.; Rahman, A.; Stock, P.; Schiano, T.D. Vitamin D Status of HIV-Positive Patients with Advanced Liver Disease Enrolled in the Solid Organ Transplantation in HIV Multi-Site Study. Liver Transplant. 2014, 20, 156–164.
- Costiniuk, C.T.; Jenabian, M.-A. Cannabinoids and Inflammation: Implications for People Living with HIV. AIDS 2019, 33, 2273–2288.
- Barré, T.; Sogni, P.; Zaegel-Faucher, O.; Wittkop, L.; Marcellin, F.; Carrieri, P.; Gervais, A.; Levier, A.; Rosenthal, E.; Salmon-Céron, D.; et al. Cannabis Use as a Protective Factor Against Overweight in HIV-Hepatitis C Virus Co-Infected People (ANRS CO13 HEPAVIH Cohort). AIDS Educ. Prev. 2022, 34, 272–290.
- Carrieri, M.P.; Serfaty, L.; Vilotitch, A.; Winnock, M.; Poizot-Martin, I.; Loko, M.-A.; Lions, C.; Lascoux-Combe, C.; Roux, P.; Salmon-Ceron, D.; et al. Cannabis Use and Reduced Risk of Insulin Resistance in HIV-HCV Infected Patients: A Longitudinal Analysis (ANRS CO13 HEPAVIH). Clin. Infect. Dis. 2015, 61, 40–48.
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147.
- Mboumba Bouassa, R.-S.; Needham, J.; Nohynek, D.; Singer, J.; Lee, T.; Bobeuf, F.; Samarani, S.; Del Balso, L.; Paisible, N.; Vertzagias, C.; et al. Safety and Tolerability of Oral Cannabinoids in People Living with HIV on Long-Term ART: A Randomized, Open-Label, Interventional Pilot Clinical Trial (CTNPT 028). Biomedicines 2022, 10, 3168.
- Canada, H. Household Food Insecurity in Canada: Overview. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/health-nutrition-surveys/canadian-community-health-survey-cchs/household-food-insecurity-canada-overview.html (accessed on 9 February 2023).
- Gregory, C.A. Food Insecurity, Chronic Disease, and Health Among Working-Age Adults. Available online: https://ageconsearch.umn.edu/record/261813/ (accessed on 20 February 2023).
- Nkambule, S.J.; Moodley, I.; Kuupiel, D.; Mashamba-Thompson, T.P. Association between Food Insecurity and Key Metabolic Risk Factors for Diet-Sensitive Non-Communicable Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 5178.
- Kardashian, A.; Serper, M.; Terrault, N.; Nephew, L.D. Health Disparities in Chronic Liver Disease. Hepatology 2022. early view.
- Golovaty, I.; Tien, P.C.; Price, J.C.; Sheira, L.; Seligman, H.; Weiser, S.D. Food Insecurity May Be an Independent Risk Factor Associated with Nonalcoholic Fatty Liver Disease among Low-Income Adults in the United States. J. Nutr. 2020, 150, 91–98.
- Kardashian, A.; Dodge, J.L.; Terrault, N.A. Food Insecurity Is Associated with Mortality Among U.S. Adults with Nonalcoholic Fatty Liver Disease and Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2022, 20, 2790–2799.e4.
- Pellowski, J.A.; Kalichman, S.C.; Matthews, K.A.; Adler, N. A Pandemic of the Poor: Social Disadvantage and the U.S. HIV Epidemic. Am. Psychol. 2013, 68, 197–209.
- Muhammad, J.N.; Fernandez, J.R.; Clay, O.J.; Saag, M.S.; Overton, E.T.; Willig, A.L. Associations of Food Insecurity and Psychosocial Measures with Diet Quality in Adults Aging with HIV. AIDS Care 2019, 31, 554–562.
- Tamargo, J.A.; Sherman, K.E.; Campa, A.; Martinez, S.S.; Li, T.; Hernandez, J.; Teeman, C.; Mandler, R.N.; Chen, J.; Ehman, R.L.; et al. Food Insecurity Is Associated with Magnetic Resonance–Determined Nonalcoholic Fatty Liver and Liver Fibrosis in Low-Income, Middle-Aged Adults with and without HIV. Am. J. Clin. Nutr. 2021, 113, 593–601.
More
