Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alvin, Chun Man Kwok and Version 2 by Catherine Yang.
Dinoflagellates are a major aquatic protist group with amphiesma, multiple cortical membranous “cell wall” layers that contain large circum-cortical alveolar sacs (AVs). AVs undergo extensive remodeling during cell- and life-cycle transitions, including ecdysal cysts (ECs) and resting cysts that are important in some harmful algal bloom initiation–termination. AVs are large cortical vesicular compartments, within which are elaborate cellulosic thecal plates (CTPs), in thecate species, and the pellicular layer (PL). AV-CTPs provide cellular mechanical protection and are targets of vesicular transport that are replaced during EC-swarmer cell transition, or with increased deposition during the cellular growth cycle.
- cell wall
- harmful algal blooms
- cyst
- dinoflagellates
- amphiesma
1. Polysaccharide Deposition during Amphiesma Dynamics
Cellulose, comprising parallel unbranched β-1, 4-linked glucan chains that form microfibrils, is the major reinforcing element of plant cell walls that provides mechanical strength [1][92]. Dinoflagellate cellulose synthase dCesA1 knockdown led to cessation of ecdysal-swarmer regeneration [2][37], without flagella, suggesting cellulose synthesis dependency in the completion of amphiesma development.
C. cohnii amphiesma was stained positively for polysaccharides (CFW staining) but negatively for callose (aniline blue staining) [3][60]. The stringent chemical assay with the Updegraff protocol [4][93] demonstrated acid-resistant crystalline cellulose content being proportional to the CFW fluorescent signals (which also stained amorphous cellulose) [3][60], supporting the CTP nanomechanical hardness [5][62]. Earlier histochemical investigations using IKI/H2SO4 and zinc–chlor–iodide (Schultz solution), glucan assays with phenol sulfuric methods, and dissolution of isolated amphiesma preparations using basic solvents (e.g., 3%-NaOH, 100 °C for 5 h) [6][7][8][8,32,94] should be reinvestigated with more stringent assays, especially in relation to the co-staining of PLs.
In Scrippsiella hexapraecingula TEM preparations, the amphiesma was positively labeled with gold conjugated-CBHI (cellobiohydrolase I, source not mentioned, likely from Trichoderma reesei) and exhibited a cellulose type electron diffraction pattern [9][10][42,44]. Many cellulose-binding domain (CBDs), including bacterial CBDs (family II CEX from Cellulomonas fimi) and single CBHI CBD and single CBHII CBD from Trichoderma reesei, also bind chitin [11][12][13][95,96,97], and many “cellulase” preparations contained other hydrolase activities [14][15][98,99]. ThWe researchers further presented here CTP/PL binding with specific cellulose-binding domain (CBHI and CBDII CBDs, [12][13][96,97]) (Figure 18A–E). General polysaccharide dyes, including CFW, will not have this distinguishing staining. Ultrastructural studies concerning amphiesmal polysaccharides were interpreted from “electron dense materials” that could have been targeted to either the PL or the CTPs. TEM studies on L. polyedrum CTP biogenesis were reviewed in [16][10].
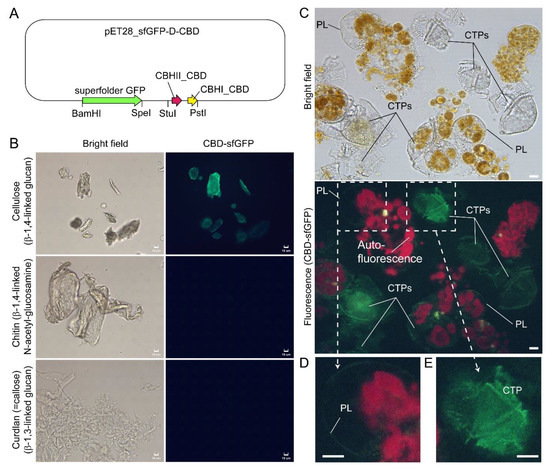
Figure 18. Compressed cell preparation of Lingulodinium polyedrum labeled with fluorescent recombinant cellulose-specific hybrid cellulose-binding domains. (A) Map of plasmid construct used for the generation of recombinant cellulose-specific carbohydrate-binding domain (CBD)-sfGFP fusion protein, which contained double CBDs as described in [13][97]. Neither single T. reesei CBHII CBD nor T. reesei CBHI CBD exhibited such specificity [12][13][96,97]. Double CBD protein was constructed by fusing the N-terminal (25–62 amino acids) of Trichoderma reesei CBHII CBD (AAG39980.1) to the C-terminal (478–513 amino acids) of T. reesei CBHI CBD (P62695.1) by a linker region of 24 amino acids (3 amino acid residues from natural CBHII linker followed by 21 amino acid residues from the natural CBHI linker). Fluorescence photomicrographs of CBD-sfGFP stained (B) microcrystalline cellulose, chitin, curdlan, and (C) Lingulodinium polyedrum cells (squashed gently). CTPs and pellicle (PL) were differentially stained green. Scale bar = 10 µm. (D,E) show higher-magnification views of the CBD-sfGFP-labelled (green) PL and CTP, respectively. These experiments also suggested previous single carbohydrate-binding domain non-specificity, of TEM CBD-gold labeling conducted in Scrippsiella hexapraecingula [9][10][42,44], could have labeled the PL. CTPs were strongly labeled, whereas PL was not labeled except along the broken rim and after extended exposure, which could be related to hydrophobic accumulation of the CBD domains that interact mainly by hydrophobicity.
Given the highly dynamic nature of amphiesmal membranes, the strict interpretation of cytoplasmic membrane(s), should be taken with caution as to the transiency of all developing stages, as well as to whether thinly deposited membrane(s) commence with polysaccharide deposition. Key CTP biogenesis issues are the synthesis of non-round polygonal regularity with taxonomic precision being orchestrated with normal and apolar cellular growth.
Plant cell non-cellulose polysaccharides are pre-synthesized in Golgi prior to transport and exocytotic deposition [17][100]. Dinoflagellate amphiesma precursors were considered to originate from some small electron-dense cortical amphisomal vesicles, which moved to the periphery of the cell, flattened, and fused together [18][19][20][33,57,59]. CFW readily stained CTPs/amphiesma of Alexandrium (Gonyaulax) tamarensis but did not label internal compartments [21][101]. Similarly, the lack of CFW staining in any intracellular compartments in C. cohnii and L. polyedrum, except in AV and PL [2][3][37,60], indicated there were no or undetectable matrix polysaccharides in the vesicular transport pathway.
Plant membrane-targeted cellulose synthases complexes (CSCs) catalyze glucose polymerization from the substrate UDP-glucose into cellulose polymer. The rosette CSC archetypes originated late in the chlorophyte lineage, whereas the linear archetypes remained in the non-green lineages [22][23][102,103], as was reported in dinoflagellate Scrippsiella hexapraecingula (although single CBD domains were deployed) [9][10][42,44]. The prominent CTPs and availability of the cyst-generation method [24][25][26][43,47,52], in combination with CFW-assisted flow cytometry of cellulose content in dinoflagellate cells [3][60], facilitated biochemical investigations of cellulose synthesis (CS) dynamics during cyst-swarmer cells transition (Tc-s) in L. polyedrum. Dinoflagellate LpCesA1 transcript was upregulated 14-fold in the early stages of ecdysal cyst regeneration, with CTPs fully regenerated between 12 and 16 h [2][37]. LpCesA1 antisense knockdown in L. polyedrum led to abnormal thecal plate deposition and postponement of the swarmer cell regeneration [2][37].
2. Amphiesma Dynamics and Vesicular Transport
Polysaccharide deposition requires vesicular transport of either in-vesicle pre-synthesis or vesicular transported cellulose synthase (CesA) that mediated on-plasma-membrane biogenesis [27][106]. Ultrastructural studies suggested polyvesicular bodies (PVBs, large endosomes) commonly located close to or attached to the alveolar sacs [10][28][44,107] with fusion of these vesicles with CM constituting amphiesmal biogenesis [29][108].
The highly dynamic amphiesma with vesicular transport was demonstrated in the polyethylene glycol (PEG)-treatment of on-agar coccoidal cells [30][109] (Figure 29A–D) with which membranous layers appeared displaced when compared to control cells. Coerced cortical membrane fusion (Figure 29B) was observed with accelerated vesicular transport resulting in dramatic amphiesmal rearrangements [30][109], demonstrating the non-permanent amphiesmal nature with sustained vesicular transport dynamics.
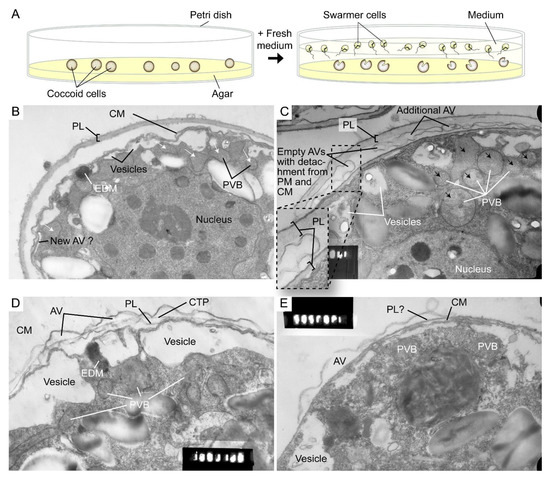
Figure 29. Amphiesmal rearrangements in coccoid cells after induced membrane fusion. (A) Schematic diagram showing the swarmer (daughter) and (mother) coccoid Crypthecodinium cohnii cells obtained by the coccoid-swarmer-release and filtration method [31][118]. For polyethylene glycol (PEG) treatment, cells were resuspended in 20% (w/v) PEG, before being spread on MLH agar plates. Transmission electron photomicrographs of the amphiesma in (B,C) coccoid (on agar plate) and (D,E) swarmer C. cohnii cells. Amphiesma of (B) control coccoid cell; (C) A PEG-treated coccoid cell; (D) A control swarmer cell and (E) swarmer cell released on PEG-treated plate. PEG treatment, which increased membrane fusion events [30][109], led to increased appearances of larger PVBs (polyvesicular bodies, large endosomes, black arrows in (C)) comparing to the smaller vesicles (white arrows in (B)) in control coccoid cells. It also drove thicker pellicular layer (PL) and amphiesmal rearrangement in the PEG-treated coccoid cell (C). The PL in PEG-treated mother cell exhibited a variation from apparently one layer with polysaccharide deposition (left) to two separate membranous layers with inter vesicular bodies (unfused, right); there were also lesser stained attached vesicular bodies outside the cell. TEM sections were in the same series that were published [30][109] and examined with a JEOL 100CX transmission electron microscope. EDM—electron dense materials. Magnification = 19,000×.
The coerced increase in fusion events [30][109] drove the disappearance of small vesicles and the accumulation of dense material in daughter swarmer cells, demonstrating the continuum of amphiesmal dynamics with the vesicular system in mother–daughter amphiesmal transition (Figure 29C,D). The small vesicles in the control cells were shifted to large peri-vesicles (~4.7 times increase in volume, as measured by ImagJ) in PEG-treated cells (Figure 29A,B). The coerced fusion of the outer layers (Figure 29D) exhibited similarity to the zooxanthellae cell wall in hospite [32][110]. PEG-treated mother cells exhibited a PL thickness variation within the same cell, from apparently one layer (left) to two separate membranous layers with inter vesicular bodies (unfused, right) (Figure 29B), indicating PL deposition involving two membranes. There were also lesser electron dense attached vesicular bodies outside the cell, substantiating the effect of extracellular PEG in driving vesicular transport, and seconding the potential role of secretion (e.g., muco-polysaccharides) in driving vesicular transport through the decanting of cortical vesicular membranes.
Lysosensor probes, which are highly pH-sensitive, strongly labeled dinoflagellate cortices coinciding with the amphiesma (Figure 310B). Smaller G1 cells appeared to have less cortical labeling when compared to the larger G2 cells (Figure 310C–E) [33][111]. pH gradients are an important regulatory axis in the vesicular transport/secretary pathway, affecting all aspects including cargo sorting and protein processing [34][35][36][37][112,113,114,115], indicating the amphiesma’s acidic pH could act as a cellular growth-deposition driver. The association of CTC[Ca2+]S (next section) further indicated amphiesma as a major homeostatic hub, having biochemical–biomechanical interactomes between the extracellular and intracellular environments. ThWe researchers do not adopt acidocalcisomes to emphasize the compartments likely different from vacuolar regulation, as lysotracker and CTC staining may not fully overlap (Figure 310A,B). The balancing of growth, with vesicular transport, with ecdysis-attrition through secretion and oxidative potentials, will be most evident in cells with apolar–circumpolar vesicular deposition.
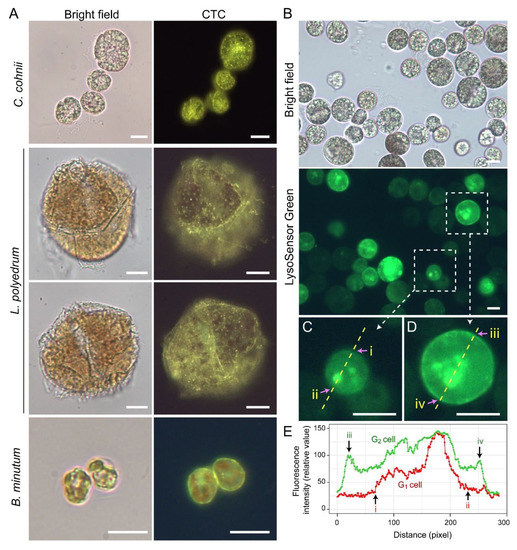
Figure 310. Amphiesma calcium stores and acidic compartments. (A) Fluorescence photomicrographs of chlorotetracycline (CTC)-stained Crypthecodinium cohnii, Lingulodinium polyedrum and Breviolum minutum cells. Cells were briefly fixed with 1% (w/v) glutaraldehyde in seawater (5 min, 22 °C) before CTC (excitation: 380 nm, emission: 520 nm [38][119]) staining with brief fixation protocol [39][120]. Over-fixation will lead to diminishing of subcellular CTC staining, suggesting the Ca2+ stores were associated with active vesicular transport. CTC-positive stores were observed on the surface and distributed over the cortical layer of the cell. In addition to the tiny-dots staining pattern, CTC also stained a continuous layer in the amphiesma (yellowish-green color). CTC localization in amphiesma could be affected by inter-membrane zeta-potential and may not specially require specific Ca2+ binding proteins. The red fluorescence is chlorophyll autofluorescence from chloroplasts. (B) LysoSensor Green DND-189 (excitation: 443 nm, emission: 505 nm, 2 µM, ThermoFisher) staining yielded fewer, but larger, dots/patchy labeling in C. cohnii. Both cell surface and subcellular compartments were stained, with apparent increased cortical labeling in larger G2 cells. (C,D) show higher-magnification views of a smaller G1 and larger G2 LysoSensor-stained cells, respectively. The boundaries of the G1 cell shown in (C) and G2 cell shown in (D) were marked by (i, ii) and (iii, iv), respectively. (E) Quantification of fluorescent level along transects in (C,D). Smaller G1 cells appeared to have less cortical labeling when compared to the larger G2 cells. In either case, there were associations of inner acid compartment with the nucleus. Scale bar = 10 µm.
Microtubules are believed to play a role in thecal development [3][24][43,60], despite there are no cortical MTOCs and the cell exhibiting no apparent dynamics; they likely form a network with alveolin homologues as reported in other Alveolates [40][41][42][14,116,117]. Amphiesma were shed in DCB-treated dinoflagellate cells [3] (Figure 2D) [60], an inhibitor of cellulose deposition through severing microtubular contact [43][82]. Actin cytoskeleton was involved in plant cellulose deposition, but cytochalasin D, an actin polymerization inhibitor, exhibited no effect in the C. cohnii cell growth progress (Chongping Li, unpublished data). The eleutheroschisis lack of unidirectional cytosol expansion, as required in desmoschisis, could thus directly reflect growth–vesicular transport through the whole genome-growth cycle, as there is no nuclear envelope breakdown. This was demonstrated with extracellular PEG coercing amphiesmal cortical layer emptying, rather than a selective increase in AV board thickness, suggesting the dynamic amphiesmal with exocytotic vesicular movement directly drives intracellular movement of vesicles (PVBs), the depletion of which led to empty AVs with detachment from the plasma membrane and the cytoplasmic membrane (Figure 29).
3. Calcium Signaling in Ecdysis, Cellular Growth and Bioluminescence
Cellular growth rate-dependent cADPR-Ca2+ signaling pathways, including dose-dependent CTC[Ca2+]S depletion, were demonstrated to orchestrate relative dinoflagellate cell growth, whereas cADPR-Ca2-store depletion mediated cortical mechanical sensitivity in dinoflagellates [39][44][78,120]. CTC[Ca2+]S mobilization exhibited pharmacological characteristics of the ciliate subplasmalemmal-like Ca2+ stores, a special cortical endoplasmic reticulum [45][46][121,122] that exhibits Ca2+ level restraint overflow from external rise [39][47][120,123]. IP3-Ca2+ signaling inhibition led to ecdysis in dinoflagellate cells [48][49][50][124,125,126], whereas Dantrolene (antagonist of both Ryanodine (RyR) and IP3 receptors) efficiently blocked shaking (caffeine)-induced Ca2+ transient. Caffeine (cADPR receptor agonist) dose-dependently accelerated Ca2+ transient and plasma membrane deposition, resulting in an increase in relative cell sizes [39][120]. Whereas cADPR activates Ca2+- SERCA to Ca2+ influx from cytosol, cADPR and inositol 1,4,5-trisphosphate (IP3) commonly operate with sensitizing luminal Ca2+ gating of RyRs/IP3R to store overload-induced Ca2+ release (SOICR) [51][52][127,128]. Inhibition of either one will modulate the other [53][54][55][129,130,131].
A dinoflagellate proton ATPase kHV1, which operated with negative Nernst potential [56][57][132,133], was proposed to function in the activation of the amphiesma associated scintillons-bioluminescence (Figure 411). Mechanically induced calcium release from intracellular Ca2+ store acts through the L-type Ca2+ channel (Figure 411), indicating the circuitry of vesicular H+-ATPase and L-type Ca2+ channels, as was shaking induced bioluminescence and mechanically induced ecdysis [28][58][59][107,134,135]. PLC inhibitor U73 122 blocked mechanically induced bioluminescence and indoleamine-induced IP3 production in dinoflagellate cells [50][60][126,136], indicating also the IP3 signaling involvement.
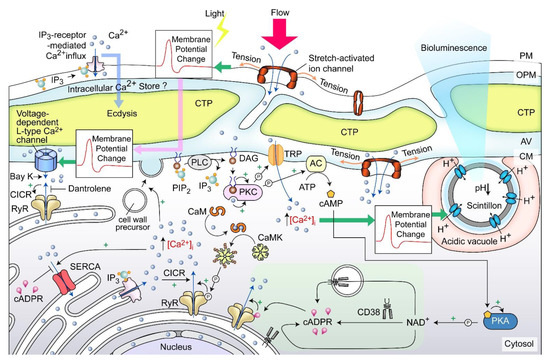
Figure 411. Amphiesma and calcium signaling. A diagrammatic representation illustrating theour observations and hypothetical amphiesmal Ca2+ signaling pathway. The positions of the scintillon and the CTC-positive Ca2+ stores are arbitrary. RyR—Ryanodine receptor; PIP2—phosphatidylinositol 4,5-bisphosphate; IP3—nositol-1,4,5-trisphosphate; IP3R—IP3 receptor; DAG—diacylglycerol; SERCA—sarco/endoplasmic reticulum Ca2+-ATPase; CICR—calcium-induced calcium release; CaM—calmodulin; CaMK—Ca2+/calmodulin-dependent protein kinase; cADPR—cyclic ADP-ribose; CD38—ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase; TRP—Transient receptor potential; cAMP—cyclic AMP; PKC—phospholipase C; AC—PKA—phospholipase A, ⓟ—phosphate/phosphorylation.
Mechanical shaking or the presence of fluidic mechanical forces inhibited cell proliferation of many dinoflagellates [61][62][63][137,138,139]. Each CTP within the surface orthogonal network of the amphiesma, with underlain cortical microtubules likely part of the mechanical sensitive system (as discussed earlier) responsible for sensing flow direction [64][140], and sustained stimulation could lead to depolarization, and, in turn, ecdysis or bioluminescence. This has similarity to the ciliate cortical AV-trichocyst system that is also based on AV Ca2+ signaling, in regulating cilia beating, including reverse swimming direction [65][66][141,142]. The intertwining between ecdysis, cellular growth, and scintillons indicates a potential bioluminescence role in dissipating oxidative stresses, as was proposed in the “oxygen defense” hypothesis [67][143].
