Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Zhengchun Liu.
Detection systems using optical principles for ion sensing have become widely used. Optical detection typically relies on the color change resulting from the interaction or reaction between the focal object and the detection reagent. Optical sensors offer the advantages of simplicity and low cost. At the same time, there is no direct contact between the sensor and the sample during analysis, thereby minimizing the effects of contamination of the sensing probe. The portable optical sensors highlighted in this section include fluorescent, colorimetric, Raman scattering, surface plasmon resonance (SPR), and localized surface plasmon resonance (LSPR) sensors.
- heavy metal ions
- portable sensing
- optical method
1. Portable Fluorescence Sensing
The basic principle of fluorescent sensors involves analyzing the content of the substance to be measured according to changes in the physicochemical properties of the fluorescent groups after the reaction between the fluorescent molecules and the detection target. These properties include fluorescence intensity and lifetime and are mainly related to charge transfer and energy transfer processes [37,38][1][2]. Typically, fluorescent sensors consist of fluorescent and receptor elements that bind specifically to the focal ion. These sensors have received considerable attention because of their low cost, fast detection, wide response range, and simplicity of operation [5,28,39][3][4][5].
Gil et al. [40][6] performed the fluorescence determination of Hg2+ in water and fish samples using a rhodamine 6G derivative-based chemical dosimeter (FC1) and a portable fiber optic fluorescence spectrophotometer. FC1 showed a high molar absorption coefficient and quantum yield, producing strong fluorescence emission at 555 nm, with a fluorescence intensity proportional to the amount of Hg2+ at the ng/mL level and a linear detection range of 0 to 12 ng/mL. The portable detection instrument consists of two optical fibers, a charge-coupled device (CCD) camera as the detector, collimating and focusing mirrors, a 500 lm diffraction grating, and an excitation source LED emitting 515 nm radiation with a filter, and AvaSoft 2048 software to control the instrument and correlate the captured fluorescence with the concentration.
Li et al. [41][7] designed a smartphone-based three-channel ratiometric fluorescence device for high-sensitivity sensing of Hg2+, Fe3+, and Cu2+ with detection limits of 3, 0.5, and 30 nM, respectively, according to the fluorescence burst mechanism of the three metal ions on three doped carbon quantum dots. Their proposed device is portable for field analysis use. The device also includes a rechargeable power supply, UV light source, reaction cup, shading box, reflector, and smartphone signal acquisition platform. This design eliminates the influence of light source intensity and ambient temperature on the fluorescence signal and improves the accuracy and reliability of the detection results.
The choice of non-toxic sensor materials is essential to avoid secondary contamination from toxic fluorescent dyes in field detection. Nath et al. [42][8] developed a paper-based integrated sensor device for simple Pb2+ and Cu2+ ion detection. Using lipoic acid solution as a probe to which the Pb2+ or Cu2+ to be measured is added, the fluorescence intensity of the solution decreases, and its color changes from red to blue because of the aggregation of gold nanoparticles (AuNPs). The authors achieved the detection of 1 ppb Pb2+ or Cu2+ on a Y-shaped test strip, where the Pb2+ or Cu2+ and the lipoic acid solutions were able to mix and interact sufficiently to produce a more pronounced signal.
To overcome the poor optical stability of fluorescent dyes, semiconductor quantum dots with better optical stability, high quantum yields, and long fluorescence lifetimes have been used for ion sensing. Chen et al. [43][9] investigated green, orange, and red luminescent quantum dots of CdTe covered with thioglycolic acid (TGA). Given the passivation and binding effect of Ag+ on the quantum dots, the transfer of electrons from the dots to Ag+ induces a red shift in the fluorescence spectrum and a reduction in fluorescence intensity, enabling the detection of trace amounts of Ag+. Accordingly, the authors designed a homemade portable sensing device (Figure 1a) for in situ detection. The detection system uses a 32-bit embedded microprocessor unit as the control center to convert the fluorescence signal after excitation of the UV photodiode into an electrical signal and establish a linear relationship between the voltage signal and the Ag+ concentration. The portable system has a detection limit of 5 nM and a linear detection range of 5 to 200 nM.
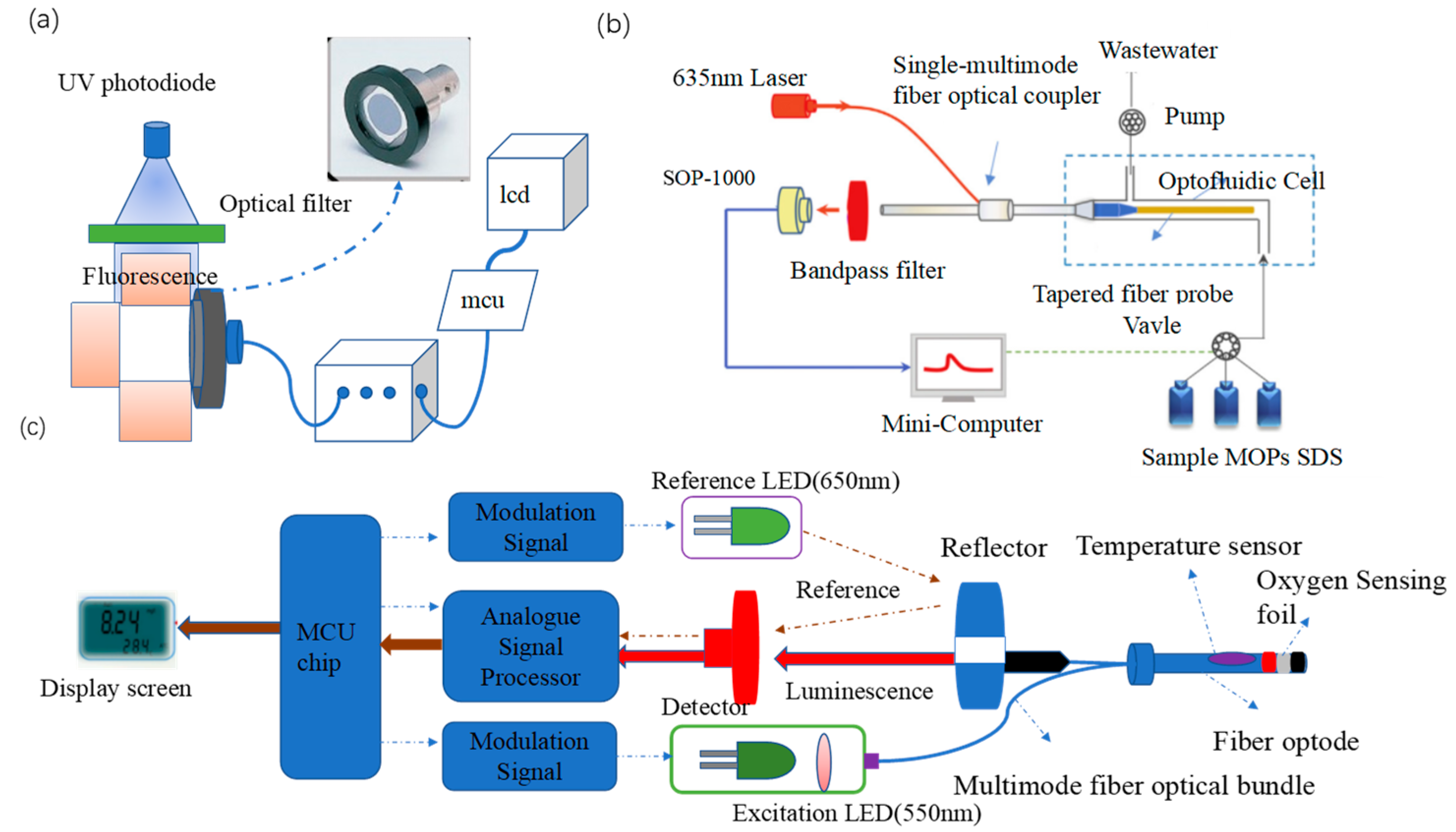
Fluorescence resonance energy transfer (FRET) is an energy transfer phenomenon between two fluorescent molecules in close proximity [46][12]. FRET can also be used for heavy metal ion detection. Sub-nanosecond anisotropic and non-linear fluorescence decay from molecular rotation are the main challenges for FRET detection, and these issues are addressed by the pulse duration and stability of laser light sources. Zhou et al. [44][10] developed a portable evanescent wave optofluidic biosensor (EWOB) with a system that uses fluorescently labeled poly-A DNA strands (CY-A14) and burster-labeled poly-T DNA strands (BQ-T14) for the highly sensitive detection of Hg2+, with a detection limit of 8.5 nM and a detection time of less than 10 min. In this platform (Figure 1b), laser light is emitted through a single multimode fiber optic coupler (SMFC) into a tapered fiber optic probe. The incident light is transmitted through the fiber probe by total internal reflection, and the electromagnetic field extending from the fiber probe interface into the solution is the evanescent wave [47][13]. The higher the Hg2+ concentration, the less CY-A14 is bound to BQ-T14, enabling the freer CY-A14 to be excited by the evanescent wave generated on the fiber optic probe surface, the stronger the fluorescence intensity detected by the EWOB.
Wang et al. [45][11] developed a novel portable whole-cell biosensing platform by integrating a simple handheld fiber-optic dissolved oxygen sensor and bacterial cultures or lyophilized bacteria. The addition of heavy metal ions inhibited E. coli respiration, enabling rapid detection of the acute toxicity of heavy metal ions. In Figure 1c, a multimode fiber optic bundle transmits excitation light and collects fluorescence, which greatly simplifies the structure of the dissolved oxygen sensor and improves the efficiency of light transmission. An O2 sensing foil containing a fluorescent oxygen-sensitive probe was applied to the end of the fiber bundle to construct the fiber node. Such an optical design achieves high portability and stability as it does not require other optical separation elements. Under optimal conditions, the detection limit and IC50 (semi-inhibitory concentration) of E. coli cultures for Hg2+ were 5.62 and 11.64 μM, respectively.
2. Portable Colorimetric Sensors
Colorimetry is a method of measuring the content of a target by comparing or calculating the color depth of colored solutions, relying on Lambert’s law and based on the production of colored compounds [48,49][14][15]. The colorimetric method provides a clear color change that can generally be identified with the naked eye. Given the simplicity and straightforwardness of colorimetric instruments, many point-of-care testing (POCT) systems for the immediate detection of non-conventional heavy metal ions are based on the colorimetric method [27,28][4][16].
Portable colorimetric sensing emphasizes smartphone integration. Xiao et al. [50][17] presented a simple, sensitive, and portable Hg2+ detection system based on a smartphone and nano-adaptor colorimetric sensor with a detection time of only 20 min. A smartphone equipped with a photometer application captures and processes the signal from the microporous reader on a smartphone (MR S-phone). The system provides linear colorimetric readings of Hg2+ concentrations in the range of 1 to 32 ng/mL, with a correlation coefficient of 0.991 and a limit of detection (LOD) of 0.28 ng/mL for Hg2+. Li et al. [51][18] developed a smartphone-based 3D printed sensing device (Figure 2a) for fast and reliable colorimetric detection. Optical sensors and light meter applications built into smartphones are used to read the test results with detection limits of 2.18 × 10−2 μg/mL, 1 μg/mL, 0.001 μg/mL, and 0.02 μg/mL for Pb2+, Hg2+, Cd2+, and Zn2+, respectively. In addition, the reagents are highly resistant to interference and are suitable for detection in real water samples.
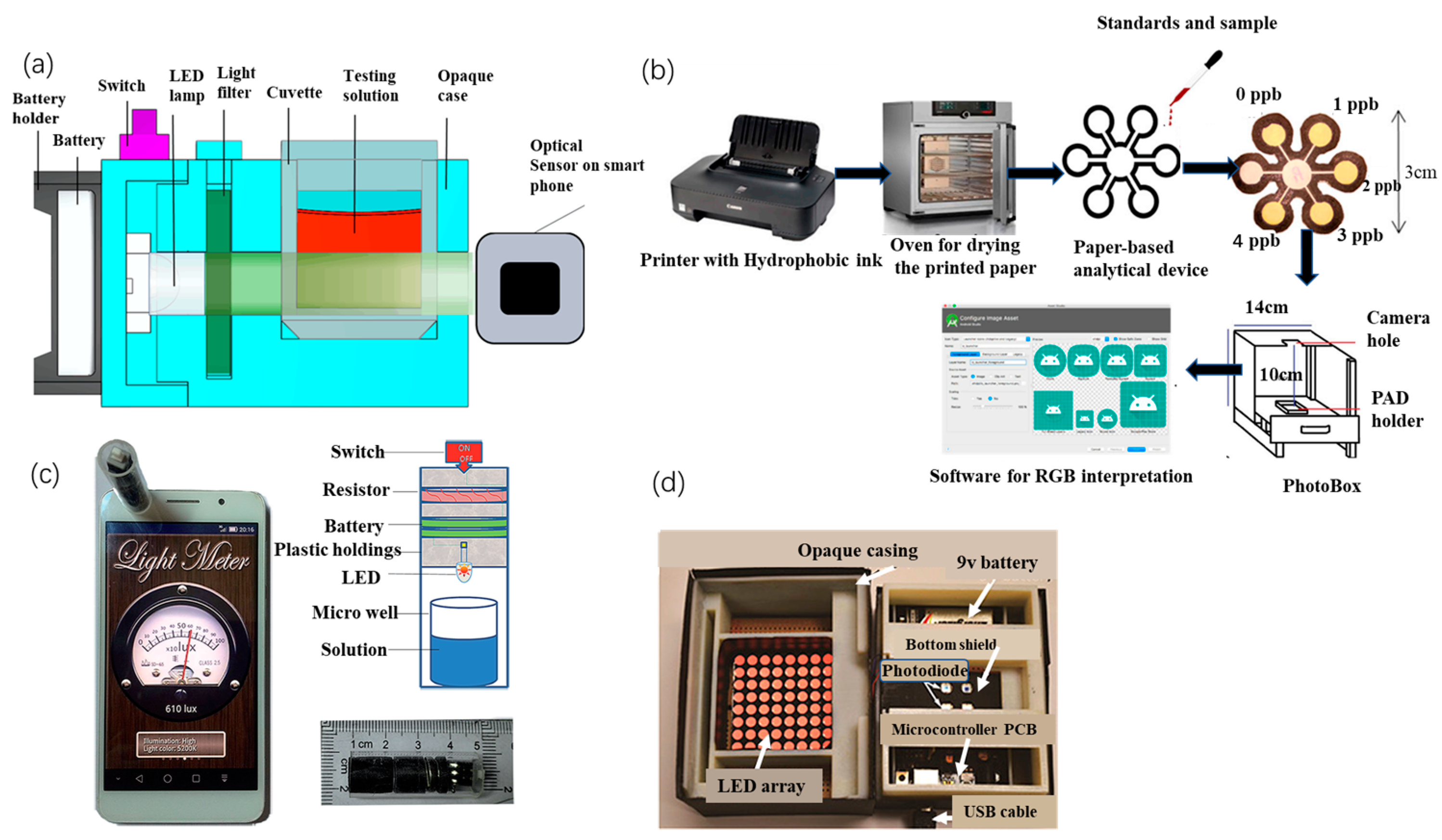
Figure 2. (a) Internal structure of the smartphone-based sensing device (SPSD) [51][18], (b) schematic diagram of the preparation and digital image acquisition of a paper-based analysis device (PAD) for the determination of Hg2+ [52][19], (c) combined smartphone and ELISA assay [53][20], and (d) inside the colorimetric reader [54][21].
Combining a smartphone with fluorescent paper strips allows instantaneous semi-quantitative detection of metal ions, significantly reducing detection time and costs. Wang et al. [55][22] developed an effective colorimetric fluorescence detection method for lead ions in water by printing paper strips with a fluorescent probe solution and using a smartphone application (color recognition) to achieve a visual, real-time, semi-quantitative detection, with a detection limit of 2.89 nM. When the test strip was immersed in the sample solution, the blue fluorescence was burst by the Pb2+ in the solution, and the red fluorescence, as a background reference, remained unchanged under UV light. A significant color change from blue to red was observed, resulting in a semi-quantitative visual detection. Subsequently, by identifying the RGB values of the fluorescent probe solution and the corresponding paper strips, the smartphone allowed the visual detection of Pb ions. Firdaus et al. [52][19] investigated a low-cost, simple, and portable method for quantitative analysis of Hg2+ based on digital image colorimetry combined with smartphone applications (Figure 2b). A small number of silver nanoparticles (AgNPs) was used as a colorimetric agent with high selectivity for Hg2+. The brownish-yellow AgNPs instantly became colorless after the addition of Hg2+ to the redox reaction. Firdaus et al. not only attached AgNPs to the media to create a paper-based analytical device but also included an Android app in the Google Play Store for data processing. The method has a detection limit of 0.86 ppb, which is comparable to the sensitivity of large conventional instruments.
The combination of a colorimetric sensing method with a photometer also enables the miniaturization of the detection device. Yu et al. [53][20] designed a portable Cr3+ assay system based on a smartphone readout device and an enzyme-linked immune-sorbent assay (ELISA) (Figure 2c). The readout device consisted of a light source and a miniaturized detection platform, and the mobile phone application “Photometer” was used to collect and process the signal from the readout device. The signal acquired by the new system is positively correlated with the Cr3+ concentration (R2 = 0.995), with a linear detection range of 0.8 to 50 ng/mL and a detection limit of 0.81 ng/mL. The system showed good selectivity in the detection of Cr3+. Camilo et al. [56][23] produced a micro-controlled photometer based on light-emitting diodes (led) using AuNPs for the detection of Pb2+. The principle of measuring Pb2+ is based on the color change in AuNPs after aggregation caused by Pb2+. The photometer uses a single LED as the light source, the sensor TCS230, an Arduino electronic card as the acquisition system, and software written in C++ to control the photometer and perform data acquisition. This system has a LOD of 0.89 mM and shows excellent selectivity for Pb2+.
In response to the low integration of the device and the low level of automation and visualization, Zhao et al. [54][21] proposed a portable analytical system based on a AuNPs probe and chip laboratory (Figure 2d). A custom microplate and a handheld colorimetric reader were designed for the colorimetric detection of Pb2+ and Al3+ in water, displaying the detection data on an integrated LCD and enabling wireless transmission of data to other devices. Calibration experiments have shown that the system achieves detection limits of 30 ppb for Pb2+ and 89 ppb for Al3+, both of which are comparable to benchtop analytical spectrometers. The colorimetric readout consists of 8 × 8 bi-color LEDs, which emit light at wavelengths of 512 to 518 nm (green) and 610 to 625 nm (red), and the microprocessor controls the emission wavelength by varying the supply voltage of the LED array. The voltage signal from the photodiode is transmitted via USB to a computer for data analysis.
3. Portable Raman Scattering Sensors
Raman spectroscopy identifies molecules by fingerprinting information from molecular vibrational spectra in analytical chemistry [57][24], biochemical sensing [58][25], and environmental monitoring [59][26]. Portable Raman scattering sensing has become an important tool for trace monitoring of environmental components and biochemical sensing because of its unique advantages of high sensitivity, unique spectral fingerprinting, and non-destructive data acquisition [60][27].
Portable Raman scattering sensing is often combined with microfluidic devices for the detection of heavy metal ions, thereby avoiding problems such as variable mixing times, scattering geometry, local heating, and photodissociation in conventional surface-enhanced Raman spectroscopy (SERS) detection under static conditions. Qi et al. [61][28] combined SERS with a microfluidic platform to achieve rapid quantitative detection of As3+ ions. Using AgNPs as a SERS-enhanced substrate, glutathione (GSH) and 4-mercaptopyridine (4-MPY) are bound to the surface of the AgNPs. When As3+ ions encounter GSH/4-MPY-functionalized AgNPs, the initially dispersed probe aggregates because of the stronger affinity of As3+ ions for GSH. Consequently, the Raman signal of 4-MPY adsorbed on the surface of AgNPs is enhanced, and As3+ ions are detected accordingly. Qi et al. designed a zigzag PDMS microfluidic channel to allow the efficient and rapid mixing of the two confluent streams, with a channel width of 350 μM and a depth of 50 μM. The linear range for the quantitative analysis of this As3+ ion sensing was 3–200 ppb, with a detection limit of 0.67 ppb.
However, this type of sensing generally requires in situ binding of the active material to the microfluidic channel, yielding a disposable sensor. To overcome this limitation, conductor-precious metal nanocomposites were used to enhance the SERS effect. He et al. [62][29] developed nucleic acid aptamer-modified sea urchin ZnO-Ag arrays that could be reused more than three times for the construction of rigid SERS biochips. In the absence of UO22+, the rhodamine B-labeled double-stranded DNA formed a rigid structure. Only a weak Raman signal was detected, but the Raman signal was amplified by pumping the UO22+ solution into the microfluidic device (Figure 3). Thus, the device can be used for ultra-sensitive and efficient detection of UO22+ ions in real environments with a detection limit of 7.2 × 10−7 μM. Similarly, to ensure the reproducibility and stability of the SERS intensity during the detection process, Zhang et al. [63][30] demonstrated an I−-functionalized SERS substrate, as I− can enhance SERS intensity by co-adsorption with a positively charged Raman reporter molecule (crystalline violet), for rapid and sensitive detection of Hg2+ on a highly integrated microfluidic platform. The sealed nature of the microfluidic device avoids interference from the environment and further improves detection efficiency and accuracy.
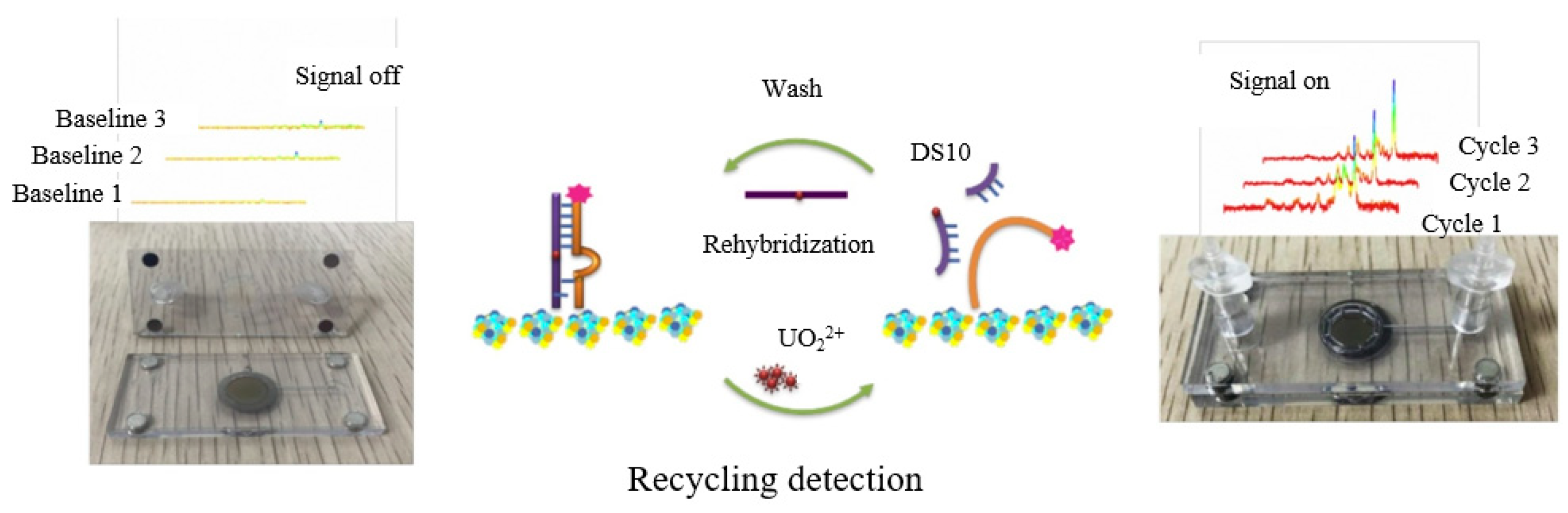
Figure 3.
Ultra-sensitive recyclable SERS microfluidic biosensor for the detection of UO
4. Local Surface Plasmon Resonance Sensing (LSPR/SPR)
The mechanism of the SPR sensor is founded on the frequency sensitivity of the oscillating electrons to the plasma nanoparticle environment [64,65][31][32]. If an electromagnetic wave (e.g., light) incident on a metal nanoparticle has a wavelength much higher than the size of the nanoparticle, then the conduction electrons will collectively begin to oscillate at a specific frequency, leading to the phenomenon known as SPR. When this collective oscillation occurs on a finite volume of particulate matter, LSPR occurs [66][33]. Both methods can be used for the rapid, accurate, and specific detection of heavy metal ions in water.
Recently, functionalized AuNPs have been used in SPR sensors, where their high refractive index and strong plasmonic absorption properties can improve detection sensitivity. Yuan et al. [67][34] developed a fiber-optic sensor for the portable and inexpensive detection of Hg2+ based on the SPR effect. They used 4-mercaptopyridine (4MPY) functionalized AuNPs (AuNPs/4-MPY) as signal amplification markers (Figure 4a), with a detection limit of 8 nM for Hg2+, under optimal conditions. Dhara et al. [68][35] used a reflection-based LSPR fiber-optic sensor to detect the concentration of Pb2+ in aqueous solutions, determined by a link between the binding rate of functionalized AuNPs immobilized on the fiber surface to Pb2+ ions and the shift of the LSPR resonance wavelength. The broadband tungsten light source employed was connected to a fiber optic coupler, and the other two ends of the fiber optic coupler were connected to the spectrophotometer and the AuNP-coated fiber. Due to the silver coating on the tip of the fiber coated with gold nanoparticles, the spectrophotometer is able to collect reflected light (Figure 4b). A PC was utilized to monitor and record the real-time absorption spectra observed in the wavelength range of 400 to 900 nm, with a sensitivity of 0.28 nm/mM for Pb2+ ions and a response time of 30 s.
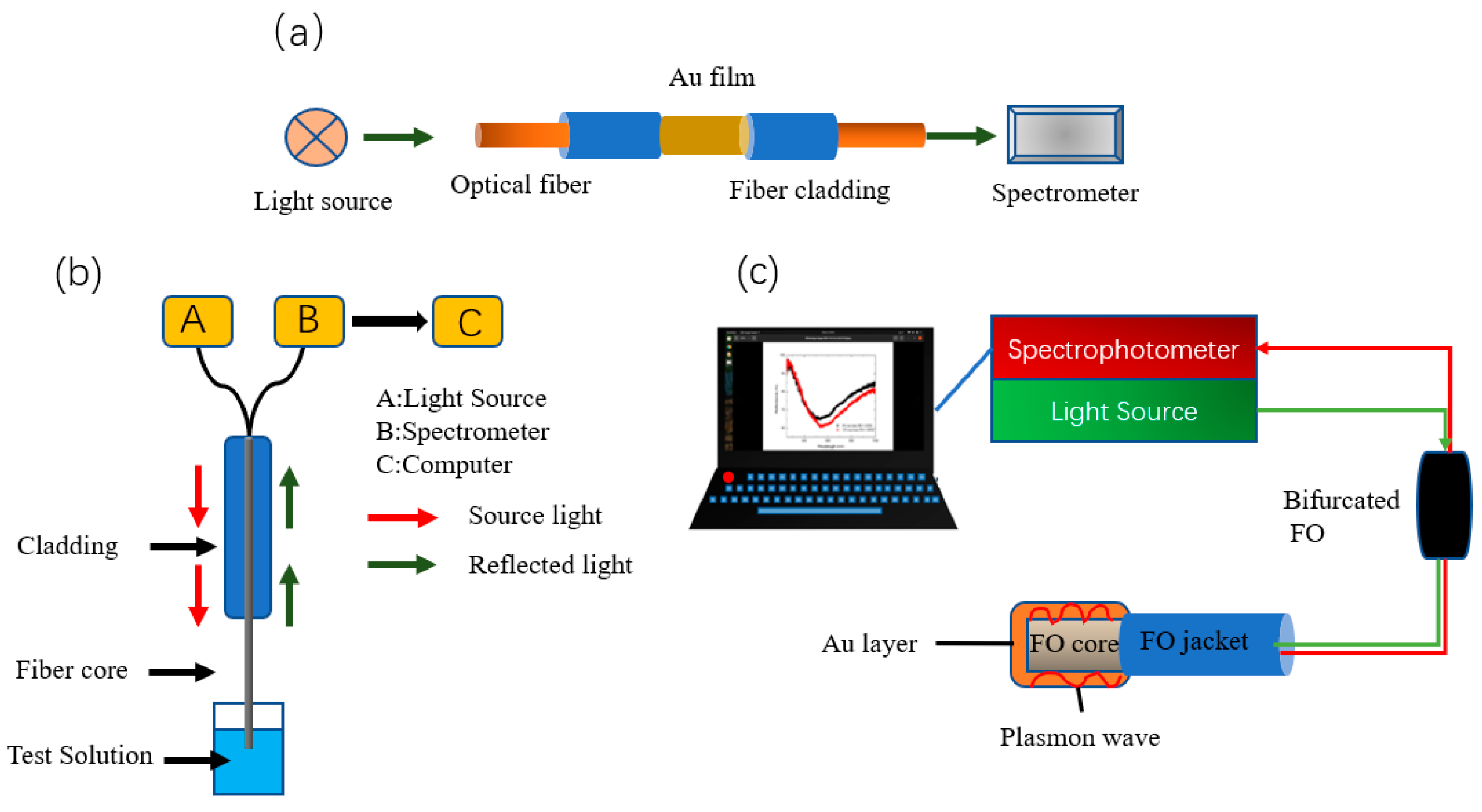
Fiber optic plasmon resonance (FO-SPR) offers the advantages of lower cost and portability compared to classical SPR devices. BG et al. [69][36] prepared a gold-plated reflective fiber-optic-surface plasmon resonance sensor, functionalized with bovine serum albumin, for the detection of cadmium ions, with a detection limit of 7.1 nM and a sensitivity of 76.67 nm/μM. The FO-SPR portable sensing system (Figure 4c) consists of a UV-Vis spectrophotometer, a tungsten halogen lamp source, a bifurcated FO, and an interchangeable FO-SPR sensor. The spectrophotometer is further connected to a laptop computer for measuring light reflected by the FO sensing head.
To achieve lower detection limits, the temperature self-compensation capability and high refractive index sensitivity of high-performance tilted fiber Bragg grating (TFBG-SPR) sensors can be exploited. Wang et al. [70][37] designed a new DNAzyme biosensor for Pb2+ detection based on the “hot spot” effect in the near-infrared band using a compact, high-performance TFBG-SPR sensing platform. The device takes advantage of the specific catalytic reaction of DNAzyme with Pb2+ and the “hot spot” effect of excitation by a narrow gap between the falling AuNPs and the metal membrane to achieve Pb2+ detection. To evaluate the utility of the TFBG-SPR biosensor, Pb2+ was added to various concentrations of tap water and clinical human serum samples. The fiber-optic biosensor was able to detect as low as 8.56 pM while obtaining a large dynamic response range of 10−11 M to 10−6 M.
References
- Sharma, P.; Bhogal, S.; Mohiuddin, I.; Yusuf, M.; Malik, A.K. Fluorescence “turn-off” sensing of iron (III) Ions utilizing pyrazoline based sensor: Experimental and computational study. J. Fluoresc. 2022, 32, 2319–2331.
- Tian, H.; Li, Z.M.; Wang, C.Z.; Xu, P.; Xu, S.F. Construction and application of molecularly imprinted fluorescence sensor. Prog. Chem. 2022, 34, 593–608.
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trac Trends Anal. Chem. 2018, 100, 155–166.
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic lab-on-a-chip platforms for environmental monitoring. Trac Trends Anal. Chem. 2017, 95, 62–68.
- Huang, G.L.; Luo, X.L.; Lin, W.M.; Tang, W.Z.; Yue, T.L.; Wang, J.L.; Li, Z.H. Carbon dots based multicolor fluorescence sensor for ratiometric and colorimetric dual-model detection of Cu2+. Dye. Pigment. 2022, 203, 110381.
- Bohoyo Gil, D.; Rodriguez-Caceres, M.I.; Hurtado-Sanchez Mdel, C.; Munoz de la Pena, A. Fluorescent determination of Hg2+ in water and fish samples using a chemodosimeter based in a Rhodamine 6G derivative and a portable fiber-optic spectrofluorimeter. Appl. Spectrosc. 2010, 64, 520–527.
- Li, D.J.; Sun, Y.; Shen, Q.R.; Zhang, Q.; Huang, W.; Kang, Q.; Shen, D.Z. Smartphone-based three-channel ratiometric fluorescent device and application in filed analysis of Hg2+, Fe3+ and Cu2+ in water samples. Microchem. J. 2020, 152, 104423.
- Nath, P.; Arun, R.K.; Chanda, N. Smart gold nanosensor for easy sensing of lead and copper ions in solution and using paper strips. RSC Adv. 2015, 5, 69024–69031.
- Chen, B.; Liu, J.; Yang, T.; Chen, L.; Hou, J.; Feng, C.; Huang, C.Z. Development of a portable device for Ag+ sensing using CdTe QDs as fluorescence probe via an electron transfer process. Talanta 2019, 191, 357–363.
- Zhou, Y.; Wang, H.L.; Song, D.; Li, Z.G.; Han, S.T.; Long, F.; Zhu, A.N. Simple, rapid, and sensitive on-site detection of Hg2+ in water samples through combining portable evanescent wave optofluidic biosensor and fluorescence resonance energy transfer principle. Anal. Chim. Acta 2021, 1155, 338351.
- Wang, H.L.; Song, D.; Chen, Y.Y.; Xu, W.J.; Han, X.Z.; Zhu, A.N.; Long, F. Development of portable whole-cell biosensing platform with lyophilized bacteria and its application for rapid on-site detection of heavy metal toxicity without pre-resuscitation. Anal. Chim. Acta 2022, 1228, 340354.
- Wang, Y.X.; Wang, N. FRET and mechanobiology. Integerative Biol. 2009, 1, 565–573.
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors: Advances of the last decade. Biosens. Bioelectron. 2016, 76, 103–112.
- Qin, M.; Li, J.S.; Song, Y.L. Toward high sensitivity: Perspective on colorimetric photonic sensors. Anal. Chem. 2022, 94, 9497–9507.
- Wang, B.T.; Niu, Y.X.; Yang, Y.; Liang, T.; Qin, X.D.; Ding, M. Sapphire Fiber High-Temperature Sensor Based on Colorimetric Method. IEEE Trans. Instrum. Meas. 2022, 71, 9508506.
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y.; et al. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends Food Sci. Technol. 2021, 109, 674–689.
- Xiao, W.; Xiao, M.; Fu, Q.Q.; Yu, S.T.; Shen, H.C.; Bian, H.F.; Tang, Y. A portable smart-phone readout device for the detection of mercury contamination based on an aptamer-assay nanosensor. Sensors 2016, 16, 1871.
- Li, B.H.; Wang, J.H.; Tu, H.H.; Yang, Z.J.; Zhao, D.F.; Feng, H.H.; Yang, J. A self-designed versatile and portable sensing device based on smart phone for colorimetric detection. Anal. Bioanal. Chem. 2021, 413, 533–541.
- Firdaus, M.; Aprian, A.; Meileza, N.; Hitsmi, M.; Elvia, R.; Rahmidar, L.; Khaydarov, R. Smartphone coupled with a paper-based colorimetric device for sensitive and portable mercury ion sensing. Chemosensors 2019, 7, 25.
- Yu, S.; Xiao, W.; Fu, Q.; Wu, Z.; Yao, C.; Shen, H.; Tang, Y. A portable chromium ion detection system based on a smartphone readout device. Anal. Methods 2016, 8, 6877–6882.
- Zhao, C.; Zhong, G.W.; Kim, D.E.; Liu, J.X.; Liu, X.Y. A portable lab-on-a-chip system for gold-nanoparticle-based colorimetric detection of metal ions in water. Biomicrofluidics 2014, 8, 052107.
- Wang, H.; Yang, L.; Chu, S.; Liu, B.; Zhang, Q.; Zou, L.; Yu, S.; Jiang, C. Semiquantitative Visual Detection of Lead Ions with a Smartphone via a Colorimetric Paper-Based Analytical Device. Anal. Chem. 2019, 91, 9292–9299.
- De L. M. de Morais, C.; Carvalho, J.C.; Sant’Anna, C.; Eugênio, M.; Gasparotto, L.H.S.; Lima, K.M.G. A low-cost microcontrolled photometer with one color recognition sensor for selective detection of Pb2+ using gold nanoparticles. Anal. Methods 2015, 7, 7917–7922.
- Fan, M.K.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29.
- Liu, X.J.; Guo, J.C.; Li, Y.; Wang, B.; Yang, S.K.; Chen, W.J.; Wu, X.G.; Guo, J.H.; Ma, X. SERS substrate fabrication for biochemical sensing: Towards point-of-care diagnostics. J. Mater. Chem. B 2021, 9, 8378–8388.
- Tang, H.B.; Zhu, C.H.; Meng, G.W.; Wu, N.Q. Review-Surface-Enhanced Raman Scattering Sensors for Food Safety and Environmental Monitoring. J. Electrochem. Soc. 2018, 165, B3098–B3118.
- Carron, K.; Ray, B.; Buller, S.; Strickland, A. New Designs for Portable Raman Instrumentation in Defense Applications. In Chemical, Biological, Radiological, Nuclear, and Explosives (Cbrne) Sensing Xvii; SPIE: Bellingham, WA, USA, 2016; Volume 9824, p. 9824.
- Qi, N.; Li, B.; You, H.; Zhang, W.; Fu, L.; Wang, Y.; Chen, L. Surface-enhanced Raman scattering on a zigzag microfluidic chip: Towards high-sensitivity detection of As(iii) ions. Anal. Methods 2014, 6, 4077–4082.
- He, X.; Zhou, X.; Liu, Y.; Wang, X.L. Ultrasensitive, recyclable and portable microfluidic surface-enhanced raman scattering (SERS) biosensor for uranyl ions detection. Sens. Actuator B-Chem. 2020, 311, 127676.
- Zhang, H.J.; Wang, D.; Zhang, D.; Zhang, T.T.; Yang, L.K.; Li, Z.P. In Situ Microfluidic SERS Chip for Ultrasensitive Hg2+ Sensing Based on I--Functionalized Silver Aggregates. Acs Appl. Mater. Interfaces 2022, 14, 2211–2218.
- Amirjani, A.; Koochak, N.N.; Haghshenas, D.F. Investigating the shape and size-dependent optical properties of silver nanostructures using UV–vis spectroscopy. J. Chem. Educ. 2019, 96, 2584–2589.
- Amirjani, A.; Sadrnezhaad, S.K. Computational electromagnetics in plasmonic nanostructures. J. Mater. Chem. C 2021, 9, 9791–9819.
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)-based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B-Chem. 2018, 273, 1768–1779.
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Liu, Q.; Qian, S.Y.; Guang, J.Y.; Wang, J.B.; Han, X.Y.; Masson, J.F.; Peng, W. Mercaptopyridine-functionalized gold nanoparticles for fiber-optic surface plasmon resonance Hg2+ sensing. ACS Sens. 2019, 4, 704–710.
- Dhara, P.; Kumar, R.; Binetti, L.; Nguyen, H.T.; Alwis, L.S.; Sun, T.; Grattan, K.T.V. Optical fiber-based heavy metal detection using the localized surface plasmon resonance technique. IEEE Sens. J. 2019, 19, 8720–8726.
- Solomonea, B.G.; Jinga, L.I.; Antohe, V.A.; Socol, G.; Antohe, I. Cadmium ions’ trace-Level detection using a portable fiber optic-surface plasmon resonance sensor. Biosensors 2022, 12, 573.
- Wang, F.; Zhang, Y.; Lu, M.D.; Du, Y.T.; Chen, M.; Meng, S.; Ji, W.; Sun, C.S.; Peng, W. Near-infrared band gold nanoparticles-Au film "hot spot" model based label-free ultratrace lead (II) ions detection via fiber SPR DNAzyme biosensor. Sens. Actuators B-Chem. 2021, 337, 129816.
More
