Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Joana Castro and Version 2 by Conner Chen.
Swine pathogenic infection caused by Escherichia coli, known as swine colibacillosis, represents an epidemiological challenge not only for animal husbandry but also for health authorities. To note, virulent E. coli strains might be transmitted, and also cause disease, in humans.
- swine colibacillosis
- AMR bacteria
- E. coli pathotypes
1. Introduction
Porcine infection caused by Escherichia coli (E. coli), so-called swine colibacillosis, is responsible for a wide range of problems, such as neonatal diarrhea, post-weaning diarrhea (PWD), edema disease (ED), septicemia, polyserositis, coliform mastitis, and urinary tract infection [1]. Among the huge diversity, certain strains of E. coli, named enterotoxigenic E. coli (ETEC), are able to cause intestinal disease, which results in neonatal diarrhea, PWD, and ED. These porcine infections are the most threatening for the swine industry worldwide due to significant economic losses associated with morbidity, mortality, decreased weight gain, the rising cost of treatments, vaccinations, and feed supplements [1][2][3][1,2,3].
PWD and ED may occur separately or together either in an individual outbreak or in the same pig [1]. Within 2–3 weeks after weaning, the piglets are more susceptible to microbial infections, owing to the existence of an immature immune system associated with sow milk removal and resulting from the interruption of the nutritive intake of immunoglobulin present in the milk [2][4][2,4]. Therefore, this period is crucial and usually associated with the most severe form of enteric E. coli infection, manifested by sudden death or severe diarrhea [1].
As an effort to promote health and growth performance, diverse approaches have been used to prevent and treat swine colibacillosis, with antibiotics being the most commonly used strategy [2][4][2,4]. Consequently, due to the growing selective pressure of antibiotic use to treat these E. coli infections, the emergence of the antimicrobial resistance (AMR) phenomenon has limited treatment options for pig producers and an increased public health concern because of the potential transfer of AMR genetic determinants directly by contact and indirectly into the food chain, water, and manure, among others [1][5][1,5]. It is important to note that E. coli has a great capacity to acquire resistance genes, mainly through horizontal gene transfer [6], in which the mobile genetic elements, such as plasmids, transposons, and gene cassettes in class 1 and class 2 integrons, seem to play a main role in the dissemination [5]. Furthermore, E. coli behaves as a donor and as a recipient of resistance genes and thus can exchange those genes with other bacteria and act as a reservoir of AMR genes [5]. Accordingly, extended-spectrum β-lactamases, carbapenemases, 16S rRNA methylases, plasmid-mediated quinolone resistance (PMQR) genes, and mcr genes constitute the most problematic genetic determinant classes of AMR in E. coli [5].
2. Etiology
According to the taxonomy, the German pediatrician Theodor Escherich (1857–1911) gave the origin to the name of the genus Escherichia. This genus belongs to the family of Enterobacteriaceae, which contemplates the Gram-negative facultatively anaerobic rods, where the species Escherichia coli fits since they are Gram-negative, peritrichously flagellated rods of variable length and a diameter of about 1 μm [1]. Over the decades, the subdivision of species into types has been carried out by the development of several classification systems. Among these, serotyping (described in Table 1) is a recognized typing system to classify E. coli strains [1]. Nevertheless, since certain porcine pathogenic E. coli belong to a limited number of serotypes, this method is less used today for diagnostic purposes [1]. Thus, the serotyping technique has been substituted by the direct detection of genes coding for bacterial determinants involved in their pathogenesis, called virulence factors. Therefore, the term pathotype is applied to the classification of E. coli typologies according to the combinations of virulence factors [3]. Common E. coli pathotypes include Shiga toxin-producing E. coli (STEC) that contains two groups, edema disease E. coli (EDEC) and enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), and extraintestinal pathogenic E. coli (ExPEC), with the most relevant E. coli pathotype in porcine, the enterotoxigenic E. coli (ETEC) [1]. It is important to highlight that EHEC and EPEC pathotypes are associated with the “attaching and effacing” (A/E) lesion development [1]. Nevertheless, EPEC is found in pigs with PWD, whereas EHEC is highly pathogenic in humans, and some zoonoses of this pathotype are sporadically recovered [1].2.1. ETEC Virulence Factors and Their Impact on Trigger Colibacillosis Infection
E. coli is both a harmless commensal bacterium in the intestines of several mammals, as well as a dangerous pathogen [5]. Still, a small proportion of strains are pathogenic and can cause severe to life-threatening intestinal and extra-intestinal infections in humans and animals [5][7][8][9][10][5,7,8,9,10]. The pathogenicity of the strains is characterized by the presence of certain virulence factor combinations in particular adhesins and toxin secretions. As it has been described, the role of adhesins and surface proteins called fimbriae is to enable the adherence of ETEC to specific receptors on the brush borders of the small intestine’s enterocytes [8]. Regarding fimbriae, there are five common antigenically different types found in pigs: F4 (K88), F5 (K99), F41, F6 (987P), and F18 [11]. The first four fimbria types are responsible for mediating adhesion in neonates, while F18 is not associated with neonatal colibacillosis; however, it is common in postweaning colibacillosis as is F4. It is also important to highlight that hemolysis is a common trait for pathogenic F4 and F18 isolates [4]. The adhesion of ETEC through fimbriae conducts the release of toxins inside the epithelial cell that promote the secretion of water and electrolytes into the intestinal lumen [1][2][8][1,2,8]. ETEC constitutes the most relevant and pathogenic strains in porcine, where the following groups of toxins are produced, namely the two major classes of enterotoxins, heat-stable toxin (ST) and heat-labile toxin (LT), as well as the enteroaggregative heat-stable toxin 1 (EAST1), as described in Table 1. STs are divided into Sta (also nominated STI, ST1, or StaP) and STb (also nominated STII or ST2) [1][2][1,2]. Similarly, LT toxins are divided into two groups, LTI and LTII. On the other hand, EAST1 is widespread among porcine ETEC, but its role in this illness remains controversial [1]. It is important to note that EAST1 alone does not seem capable of developing the disease; however, together with LT, it takes effect [1][2][1,2]. Additionally, it is important to note that Shiga toxin (Stx or VT), namely the Shiga toxin type 2e (Stx2e), E. coli is also found in some ETEC strains, being commonly found in ETEC strains expressing LT and/or ST toxins [11]. This toxin is a causative factor of edema in swine, and it has been also associated with diarrhea commonly found in colibacillosis [1][11][1,11]. Regarding the route of contamination, ETEC is firstly ingested through the oral route and then passes through the stomach. When it reaches the intestine, in the presence of suitable environmental conditions, ETEC proliferates and causes disease [1][8][1,8]. At this point, they colonize the small intestine following the attachment of fimbria adhesins to specific receptors present on the epithelium as well as in the mucus coating the epithelium of the small intestine (see Figure 1). This promotes the production and release of enterotoxins, as mentioned above, inside the epithelial cell that stimulates the secretion of water and electrolytes into the intestinal lumen, which leads to diarrhea, weight loss, and possibly death [1][3][8][1,3,8]. To note, the combinations of different ETEC virulence factors (adhesins and toxins) are associated with the development of different diseases, namely neonatal diarrhea and PWD, as shown in Table 1.Table 1.
Virulence factors of enterotoxigenic
Escherichia coli
(ETEC) associated with swine enteric colibacillosis.
| Adhesins | Toxins | Serotypes | Disease |
|---|---|---|---|
| F5, F6, F41 | STa | O8, O9, O20, O64, O101 | Neonatal diarrhea |
| F4 | STa, STb, LT, EAST1, α-hemolysin b | O8, O138, O141, O145, O147, O149, O157 | Neonatal diarrhea Diarrhea in young pigs preweaning |
| F4, AIDA a, unknown | STa, STb, LT, EAST1, α-hemolysin b | O8, O138, O139, O141, O147, O149, O157 | PWD |
| F18, AIDA a | STa, STb, LT, Stx (or VT) c, EAST1, α-hemolysin b | O8, O138, O139, O141, O147, O149, O157 | PWD |
a AIDA is a non-fimbrial adhesin involved in diffuse adherence; nevertheless, the mechanism of AIDA in swine colibacillosis is not yet elucidated. However, this non-fimbrial adhesin has been associated with ETEC strains from weaned pigs with PWD [2]. b α-hemolysin is a pore-forming cytolysin associated with ETEC strains that cause diarrhea in animals [12]. c Shiga toxins (Stx or VT) are cytotoxins produced by Shiga-toxin-producing E. coli (STEC), and in swine, the most important STECs are those that cause edema disease (ED) [1].
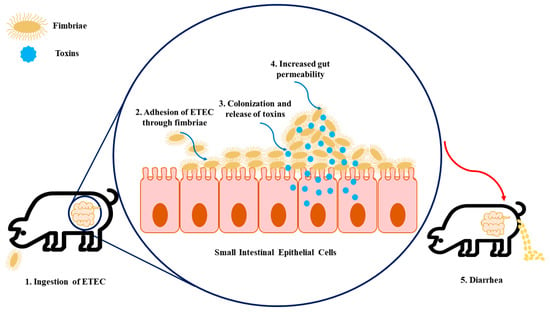

Figure 1. The infection model of enterotoxigenic Escherichia coli (ETEC) on intestinal epithelial cells. (1) Firstly, the swine ingest ETEC, enabling its transition to the gastrointestinal tract. (2) The fimbriae expressed by ETEC allow the adhesion of bacteria to specific receptors present in the intestinal epithelial cells. (3) Colonization arises in the small intestinal mucosa, which leads to the production of toxins. (4) These enterotoxins promote water and electrolyte loss into the intestinal lumen, resulting in increased gut permeability. (5) As a consequence of increased gut permeability and massive water loss, diarrhea, weight loss, and mortality can happen.
