Circulating fatty acids (FA) have an endogenous or exogenous origin and are metabolized under the effect of many enzymes. They play crucial roles in many mechanisms: cell signaling, modulation of gene expression, etc., which leads to the hypothesis that their perturbation could be the cause of disease development. FA in erythrocytes and plasma rather than dietary FA could be used as a biomarker for many diseases. Cardiovascular disease was associated with elevated trans FA and decreased DHA and EPA. Increased arachidonic acid and decreased Docosahexaenoic Acids (DHA) were associated with Alzheimer’s disease. Low Arachidonic acid and DHA are associated with neonatal morbidities and mortality. Decreased saturated fatty acids (SFA), increased monounsaturated FA (MUFA) and polyunsaturated FA (PUFA) (C18:2 n-6 and C20:3 n-6) are associated with cancer. Additionally, genetic polymorphisms in genes coding for enzymes implicated in FA metabolism are associated with disease development. FA desaturase (FADS1 and FADS2) polymorphisms are associated with Alzheimer’s disease, Acute Coronary Syndrome, Autism spectrum disorder and obesity. Polymorphisms in FA elongase (ELOVL2) are associated with Alzheimer’s disease, Autism spectrum disorder and obesity. FA-binding protein polymorphism is associated with dyslipidemia, type 2 diabetes, metabolic syndrome, obesity, hypertension, non-alcoholic fatty liver disease, peripheral atherosclerosis combined with type 2 diabetes and polycystic ovary syndrome. Acetyl-coenzyme A carboxylase polymorphisms are associated with diabetes, obesity and diabetic nephropathy. FA profile and genetic variants of proteins implicated in FA metabolism could be considered as disease biomarkers and may help with the prevention and management of diseases.
- fatty acids
- diseases
- gene polymorphisms
- red blood cells
1. Introduction
2. Fatty Acid Profile as Disease Predictor
2.1. Fatty Acids and Diseases
.1. Fatty Acids and Diseases
2.1.1. Fatty Acids and Cardiovascular Disease (CVD)
FA play a crucial role in CVD. They exert their effect by acting on lipoprotein metabolism [9][26] and endothelial function [10][27]. FA-mediated dysregulation of nitric acid and cytokine production, inflammation, oxidative stress, apoptosis, and activation of the renin–angiotensin system, which causes endothelial dysfunction, therefore increases CVD risk [10][27]. Many FA are associated, whereas others are inversely associated with CVD. A strong inverse correlation was shown between RBC oleic acid and CVD [11][28]. Another study showed a protective effect of plasma very long-chain SFA (arachidic acid (20:0), behenic acid (22:0) and lignoceric acid (24:0)) in heart failure [12][29]. However, Hadj Ahmed et al. showed an increased level of RBC C26:0, C24:0, C22:0, EPA and AA in patients with coronary artery disease compared to the normal population and consider them biomarkers of coronary artery disease [13][9]. The same team showed an association between RBC and plasma trans FA and coronary artery disease severity [14][30]. Many other studies showed an association between RBC trans FA and coronary heart disease [9][26]. This association is mediated by increased low-density lipoprotein cholesterol and decreased high-density lipoprotein cholesterol [9][26]. Acute myocardial infarction is associated with decreased short-chain FA and increased long-chain FA levels [15][31]. Acute coronary syndrome is inversely associated with DHA/AA ratio [16][32]. Early onset coronary atherosclerosis is associated with decreased EPA and DHA levels [17][33] and risk of atherosclerotic plaque rupture is associated with high AA/DHA ratio [18][34]. The results are different for different studies. For example, cardiovascular disease is associated with elevated n-6 PUFA [19][35] or elevated SFA and trans FA [20][36] or increased MUFA [21][37] and the same is true for coronary artery diseases [13][22][9,38]. Such differences could be explained by genetic, lifestyle and dietary differences between the studied populations. One study showed that the association between FA and cardiometabolic risk is modulated by concurrent physical activity [23][39]. The main conclusion is that the FA profile is modified between healthy subjects and patients. The most consistent association is for increased trans FA and decreased DHA and EPA. Moreover, the FA profile could predict cardiovascular disease development in unhealthy subjects such as diabetic patients [24][40] and patients with renal failure [25][41]. Additionally, it predicts psychiatric disorder at 6 months after acute coronary syndrome [26][42]. Moreover, it predicts mortality among patients with acute cardiovascular disease [27][43], coronary artery disease [28][44] and with myocardial infarction [29][45].2.1.2. Fatty Acids and Diabetes
Many studies have highlighted the association between elevated total plasma free FA and diabetes [30][31][32][46,47,48], whereas other studies examined individual FA. RBC n-3 PUFA was negatively associated with the risk of type 2 diabetes [33][49]. Serum C22:0 and plasma n-6/n-3 were associated with diabetes [34][35][50,51]. Many studies have agreed on elevated plasma palmitic acid, stearic acid and oleic acid in diabetic patients [31][32][36][37][38][39][47,48,52,53,54,55]. In fact, palmitic acid slows down insulin signal transduction [40][56]. Meanwhile, the association between increased oleic acid concentration and the development of diabetes is not well understood, as studies show that oleic acid may play a role in the protection and treatment of diabetes [39][55]. Elevated RBC-linolenic acid was associated with high type 2 diabetes incidence [41][57].
2.1.3. Fatty Acids and Cancer
A study in patients with any type of cancer (except head and neck cancer) showed that cancer is associated with decreased SFA (C16:0 and C18:0) and increased MUFA (C18:1) and PUFA (LA and C20:3 n-6) [42][65]. Hepatocellular carcinoma is strongly associated with low levels of very long-chain SFA and n-3 PUFA [43][66]. Oral cancer is associated with decreased EPA and DHA [44][67]. Pancreatic cancer is associated with decreased n-3 PUFA and MUFA and increased arachidonic acid [45][68], whereas the association between FA and breast cancer depends on many variables, such as menopause [46][69] and BMI [47][70]. In breast cancer, FA (omega 3 and omega 6) modulate the cancer immune response and the fatty acid endogenous synthesis [48][71]. The concentration of oleic acid is lower in cancer patients, increasing their exposure to the disease [49][72].2.1.4. Fatty Acids and Other Diseases
Serum γ-linolenic acid and C9:0 and C19:0 were associated with obesity [50][51][75,76] and dihomo-gamma-linolenic acid and palmitoleic acid were able to predict the future development of metabolic syndrome (MS) in obese subjects [52][77]. Zarrouk et al. identified hexacosanoic (C26:0) as blood (RBC and plasma) lipid biomarkers of dementia [53][7], whereas Yamagishi et al. showed that Serum ALA was inversely associated with the risk of disabling dementia [54][78]. Hammouda et al. showed that AA increased in plasma and RBC and DHA decreased only in plasma of Alzheimer patients [55][79]. Additionally, Sala-vila et al. found that DHA was inversely associated with Alzheimer’s disease [56][80]. Meanwhile, a study by Tomata et al. did not support the association between PUFA and Alzheimer’s disease risks [57][81].2.2. Polymorphisms of Gene Implicated in Fatty Acid Metabolism and Diseases
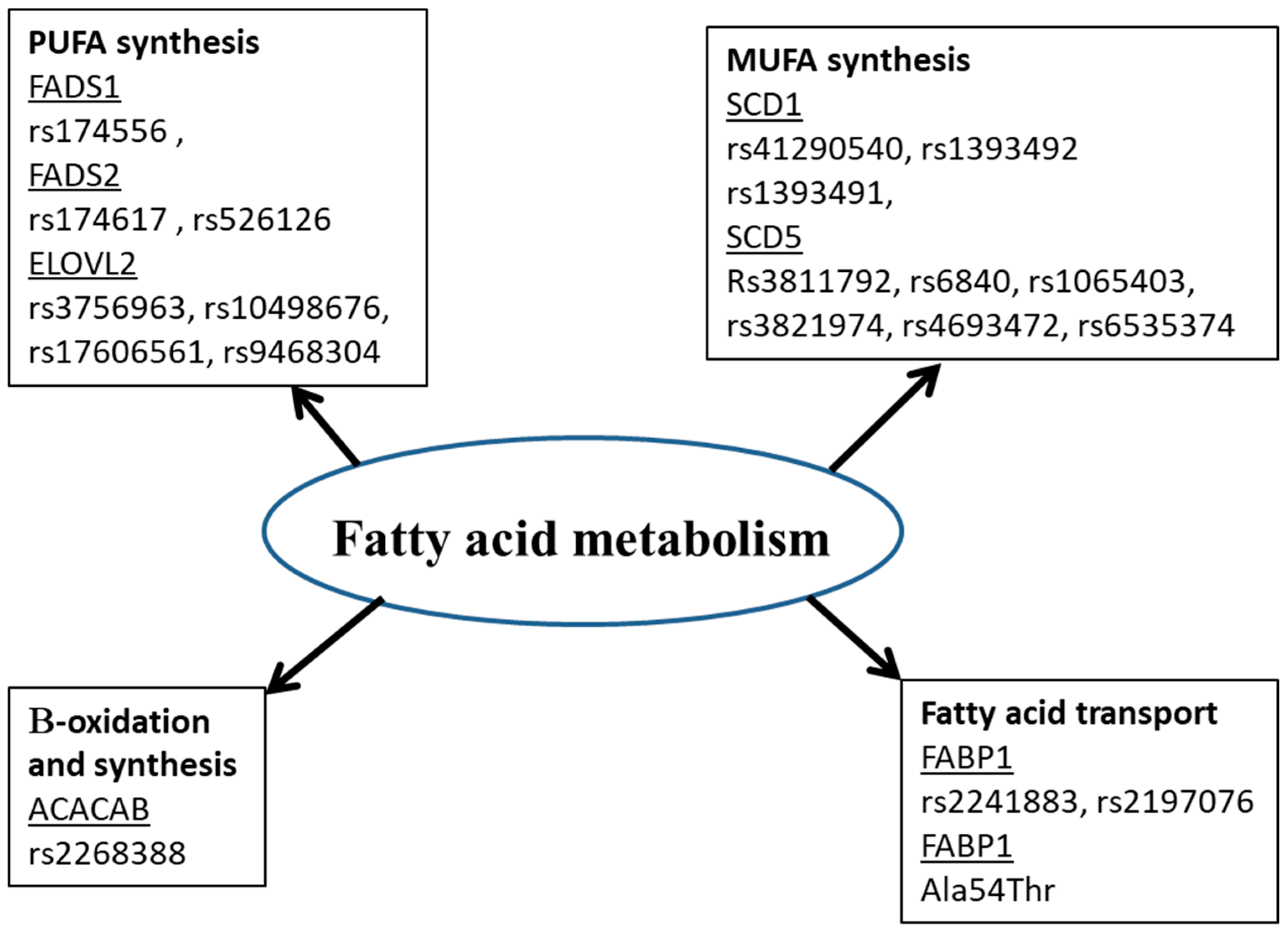
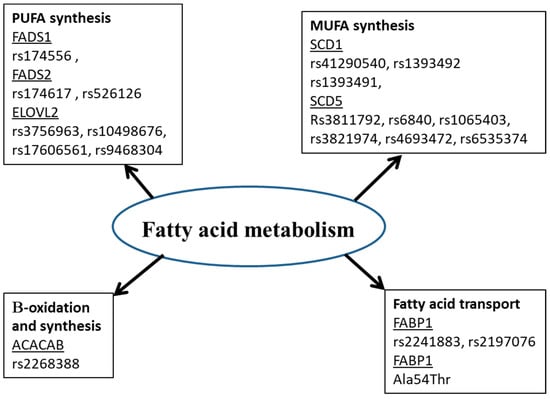
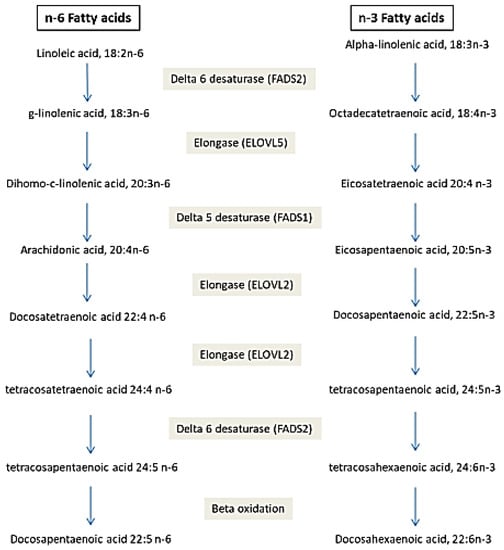
Many studies showed that the fatty acid in biological tissues is an indicator of fatty acid intake [58][59][89,90]. However, this is not always the case. Amézaga et al. showed that the variation in erythrocyte FA is not linked to dietary intake [42][65]. FA have an exogenous and endogenous origin. They can be provided by food or synthesized, mainly in hepatocytes. Several proteins are involved in the metabolism of FA (Figure 1). Any perturbation in the activity or quantity of those enzymes could be a cause of a fatty acid profile change, then disease development [60][5].