5-Aminolevulinic acid (ALA) is a naturally occurring amino acid synthesized in all nucleated mammalian cells. As a porphyrin precursor, ALA is metabolized in the heme biosynthetic pathway to produce protoporphyrin IX (PpIX), a fluorophore and photosensitizing agent. ALA administered exogenously bypasses the rate-limit step in the pathway, resulting in PpIX accumulation in tumor tissues. Such tumor-selective PpIX disposition following ALA administration has been exploited for tumor fluorescence diagnosis and photodynamic therapy (PDT) with much success. Five ALA-based drugs have now received worldwide approval and are being used for managing very common human (pre)cancerous diseases such as actinic keratosis and basal cell carcinoma or guiding the surgery of bladder cancer and high-grade gliomas, making it the most successful drug discovery and development endeavor in PDT and photodiagnosis.
1. ALA-Mediated PpIX Biosynthesis in the Heme Biosynthesis Pathway
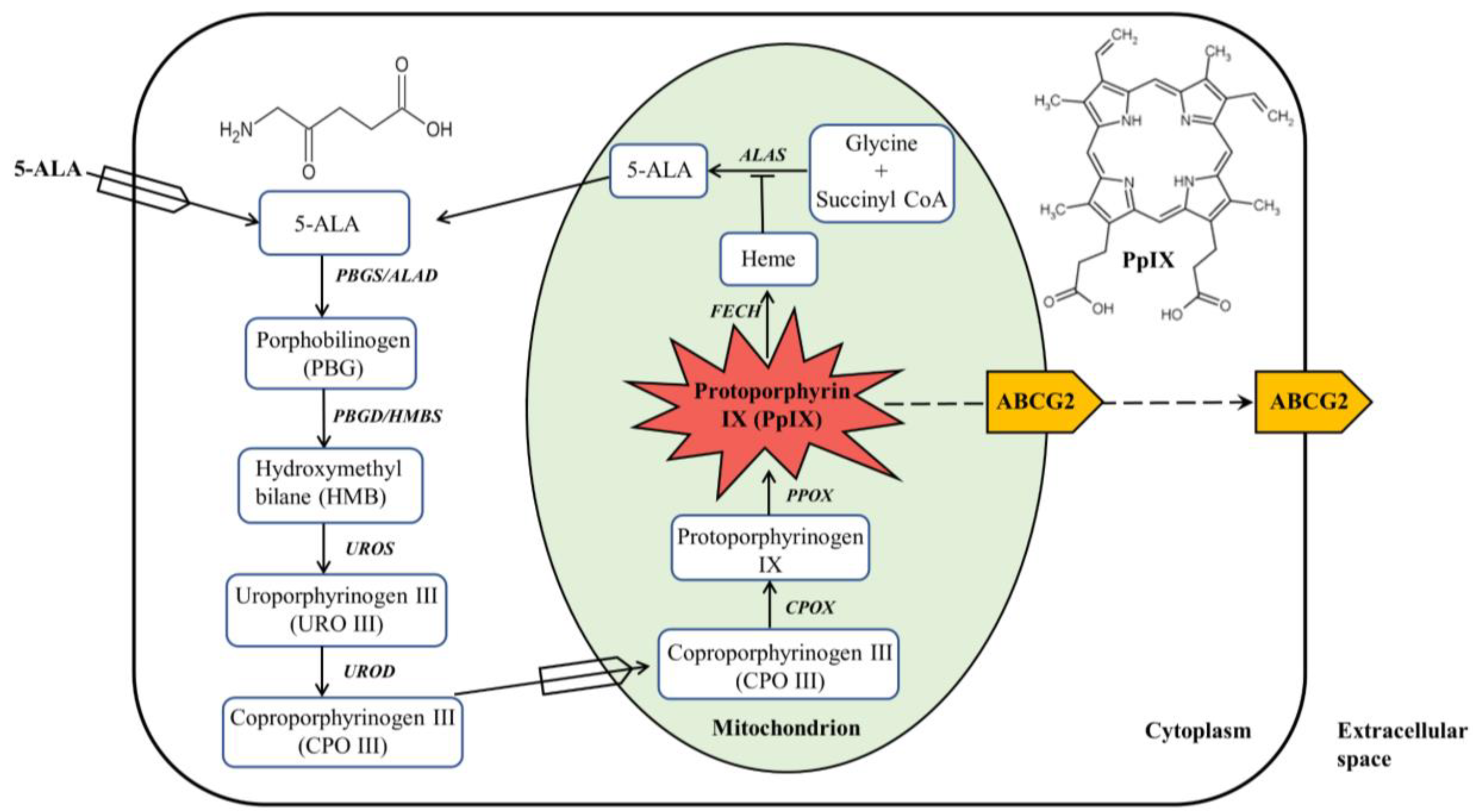
Heme biosynthesis in mammalian cells is a finely tuned process that involves eight enzymes with four in the cytoplasm and four in the mitochondrion
[8][1], as shown in
Figure 1. The first step in the heme biosynthesis pathway is the synthesis of ALA from glycine and succinyl-CoA by ALA synthase (ALAS) in the mitochondria. Succinyl-CoA is a tricarboxylic acid (TCA) cycle intermediate that is derived from α-ketoglutarate (α-KG) by α-KG dehydrogenase. Glycine is the simplest amino acid that is abundant in cells for multiple biological processes. There are two tissue-specific ALAS enzymes,
ALAS1 and
ALAS2 encoded by two different genes
ALAS1 and
ALAS2, respectively.
ALAS2 located on the X-chromosome is expressed only in the erythroid precursor cells, whereas
ALAS1 on chromosome 3 is expressed in all other types of cells. As the first intermediate in the heme biosynthesis pathway, ALA is a simple amino acid with no fluorescence and photosensitizing activity. Since the production of ALA is the rate-limiting step of heme synthesis, exogenous ALA given orally or topically bypasses this step, resulting in increased porphyrin synthesis.
Figure 1. ALA-mediated PpIX biosynthesis in the heme biosynthesis pathway. ALA administered exogenously is metabolized in the heme biosynthesis pathway, which is composed of four enzymes in the cytoplasm and four enzymes in the mitochondrion, to produce PpIX with red fluorescence and photosensitizing activity. PpIX can be converted to heme with no fluorescence and photosensitizing property or effluxed out via primarily ABCG2 transporter.
The second step of heme biosynthesis is the formation of porphobilinogen (PBG) from two molecules of ALA catalyzed by ALA dehydratase (ALAD), also called porphobilinogen synthase (PBGS). This step as well as the following three enzymatic reactions all happen in the cytosol. ALAD is a tetramer of homodimers that utilizes zinc to both stabilize the protein structure and catalyze the reaction. Once four molecules of PBG are synthesized, they are linked together via deamination to form a linear tetrapyrrole named hydroxymethylbilane (HMB) by the enzyme porphobilinogen deaminase (PBGD), also called hydroxymethylbilane synthase (HMBS). HMB will be cyclized to produce the cyclic tetrapyrrole molecule uroporphyrinogen III (URO III) by uroporphyrinogen III synthase (UROS). There is a possibility that spontaneous cyclization of the HMB molecule may occur, forming uroporphyrinogen I, which will not proceed to form heme. Decarboxylation of URO III by uroporphyrinogen decarboxylase (UROD) leads to the formation of coproporphyrinogen III (CPO III), which completes the cytosolic process of heme biosynthesis.
CPO III is then imported into the mitochondrial intermembrane space where it is converted into protoporphyrinogen IX, a reaction catalyzed by coproporphyrinogen III oxidase (CPOX). Protoporphyrinogen IX crosses the mitochondrial inner membrane and is aromatized by protoporphyrinogen oxidase (PPOX) to produce PpIX, a porphyrin metabolite with fluorescent and photosensitizing properties. Heme biosynthesis ends in the mitochondrial matrix where ferrous iron (Fe
2+) is inserted into the tetrapyrrole core of PpIX by ferrochelatase (FECH) to form heme
[8][1]. With the insertion of paramagnetic iron into PpIX, heme possesses neither fluorescence nor photosensitizing activity
[9][2].
Being a ferrous iron-containing porphyrin molecule, heme is utilized as a prosthetic group in various heme-containing proteins with important biological functions
[10][3]. In hemoglobin and myoglobin, heme functions to assist in oxygen transport and storage. In cytochromes, it functions as a catalytic group for processes such as electron transport and ATP synthesis. Additionally, heme is a catalytic necessity for enzymes involved in detoxification processes such as catalases and peroxidases
[10,11][3][4]. Heme itself is the most important regulator of the heme biosynthesis pathway in that it negatively regulates heme biosynthesis via the inhibition of ALAS and stimulates heme catabolism via activating heme oxygenase 1 (HO-1)
[12][5].
2. ALA as a Therapeutic Agent for PDT
PDT is a light-based therapeutic modality that involves a photosensitizer, a source of light with a specific wavelength, and molecular oxygen. These components individually are not harmful but become cytotoxic when combined due to the generation of reactive oxygen species (ROS) via type I and II photochemical reactions. Upon the absorption of light energy, photosensitizer molecules at the ground singlet state are excited to the excited singlet state. Molecules at the excited state are very short-lived and can decay back to the ground state by energy dissipation through fluorescence emission and internal conversion to heat, or undergo intersystem crossing to the excited triplet state with longer lifetime. Photosensitizer molecules at the triplet state may directly transfer electrons to substrate molecules to produce free radicals (Type I reaction) or, more commonly, transfer energy to molecular oxygen (Type II reaction), which is often abundantly available in the tissue of oxygen-consuming species, to generate single oxygen (
1O
2). With high reactivity, singlet oxygen can oxidize various biological substances including proteins, lipids, and nucleic acids, causing tissue oxidative damage. Due to the short lifetime and diffusion distance of singlet oxygen, the site of oxidative damage is in close proximity to the localization of the photosensitizer
[13][6]. As a non-invasive therapeutic modality, PDT with various photosensitizers has been used in clinical settings for both cancerous and non-cancerous diseases
[14][7]. The most commonly used photosensitizer is PpIX derived from exogenously applied ALA.
ALA was known to cause endogenous PpIX accumulation in human lymphocytes in the 1970s
[15][8]. In this early
res
tudyearch, ALA-induced PpIX was much higher in lymphocytes from erythropoietic protoporphyria patients (carrying the loss-of-function mutation of the
FECH gene) than in normal human lymphocytes and could be further enhanced by an iron chelator, suggesting the importance of
FECH and ferrous iron in regulating PpIX level. Photoactivation of endogenous PpIX induced by ALA results in the elimination of erythroleukemia cells as Malik et al. showed in 1987, which is the first report of using ALA-PDT for cell inactivation
[5][9]. The finding made by Kennedy et al. that ALA in aqueous solution can penetrate through abnormal keratin but not normal keratin, and induces lesion-selective PpIX in the epidermis but not in the dermis led them to conduct the first clinical trial of ALA-PDT for actinic keratosis (AK) and superficial basal cell carcinomas (BCC)
[6][10]. In 1990, they reported an almost perfect patient response rate (complete and partial response combined) and excellent cosmetic results. Their pioneering work of applying ALA topically for stimulating local PpIX production followed by aimed light illumination for cell inactivation marked the beginning of future successes of ALA-PDT for AK and superficial BCC, the most common precancerous and cancerous skin lesions, respectively.
Multiple phase II and III clinical trials followed the initial promising study by Kennedy et al. In phase III trials of 241 patients with head AK, topical application of ALA solution on the lesion area followed by blue light treatment induced a 72% complete response rate at 12 weeks after PDT, whereas only a 20% response rate was seen in patients treated with vehicle and light only
[16][11]. Patients were re-treated if lesions showed incomplete response or recurrence at 8 weeks after PDT. The recurrence rate between 8 and 12 weeks was 5% in all PDT-treated lesions. In contrast, 27.9% of lesions treated with vehicle and light only recurred during the same time period. With this excellent clinical performance, 20% ALA hydrochloride solution (Levulan) combined with blue light illuminator (centered at ~417 nm) received FDA approval for PDT treatment of AK in 1999, becoming the first ALA preparation entering the clinical application
[17][12].
To increase transdermal tissue penetration for thick skin lesions, the methyl ester of ALA methyl aminolevulinate (MAL) with higher lipophilicity than ALA was evaluated for PpIX production
[18][13]. Although MAL produces less PpIX than ALA due to the need for cleaving the ester bond to release free ALA, PpIX induced by MAL is more selective in AK lesions than that induced by ALA
[19][14]. MAL also induces selective PpIX accumulation in thick human BCC lesions following topical application
[20][15]. This
res
tudyearch also evaluated the effects of MAL dose and incubation time on the intensity and depth of PpIX fluorescence and concluded that the application of 160 mg/g MAL for 3 h provided the highest ratio of PpIX fluorescence depth to tumor depth. PDT with this MAL protocol and red light illumination (with deeper tissue penetration than blue light) showed up to 91% and 97% complete responses in AK and BCC lesions, respectively, in randomized multicenter phase III clinical trials
[21][16]. This response rate renders MAL-PDT at least equally effective as cryotherapy or excision surgery, two conventional therapies for AK and BCC. The cosmetic outcome with MAL-PDT is better than with the latter two. Based on these clinical trials, in 2004, the FDA approved PDT with 160 mg/g MAL cream (Metvixia) applied 3 h before red light (centered at ~630 nm) illumination for both thin and moderately thick AK lesions.
Another successful approach to enhancing ALA tissue penetration is to formulate it with BF-200 nano-lipid vesicles, which increases the fusion between ALA nano-vesicles and the phospholipid bilayers of skin cells
[22][17]. The resulting ALA nano-emulsion with 10% ALA hydrochloride (BF-200 ALA) not only leads to much stronger PpIX fluorescence than that by MAL cream but also induces fluorescence at a tissue depth of more than twice as much as that achieved with MAL cream. Multiple phase III clinical trials demonstrated that topical application of BF-200 ALA followed by red light irradiation 3 h later induced a significantly higher response rate in AK patients than PDT with 20% solution (Levulan) or 160 mg/g MAL cream (Metvixia)
[23][18]. In 2016, BF-200 ALA gel (Ameluz) in combination with red light illumination (centered at ~635 nm) was approved by the FDA for lesion-directed and field-directed treatment of mild-to-moderate severity AK, making it the third ALA-based preparation approved for AK treatment and the only one approved for field-directed treatment.