Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 3 by Dean Liu.
Pharmacological research has led to the development of a new drug, the bempedoic acid, which further enrich the available therapies. This drug also acts on the biosynthesis of cholesterol but at upstream level than statins. From the biochemical point of view, it has the potential to be considered before the statin with consequent titration of statins to achieve the desirable LDL-C target.
- low-density lipoproteins
- atherosclerosis
- lipid-lowering strategy
1. Introduction
The relationship between low-density lipoprotein cholesterol (LDL-C) levels and cardiovascular events, such as myocardial infarction and stroke, has been confirmed by epidemiological, clinical, and Mendelian studies [1][2]. Based on this evidence, reducing LDL-C has become a key therapeutic target in the management of cardiovascular diseases (CVD) [3][4]. Over the past two decades, the concept “The lower the better” has been established indicating that higher is the LDL-C reduction, greater will be the benefits in terms of cardiovascular events [3]. The most recent guidelines from the European Society of Cardiology (ESC) on the management of dyslipidemias have further reduced the therapeutic targets of LDL-C in patients at high and very high cardiovascular risk, suggesting the ambitious value of 70 and 55 mg/dL, respectively [5]. Furthermore, in the patients with a previous acute coronary syndrome (ACS) who undergo a further event, an LDL-C levels < 40 mg/dL is suggested, identifying a class of patients defined at extreme cardiovascular risk [6]. These targets are now achievable owing to the availability of highly effective drugs such as statins, which today represent the most prescribed first-line drugs [7] in addition to a healthy lifestyle. However, in the real world, statin use is associated with large inter-individual variability in terms of response to a fixed statin dose [8] and with the risk of intolerance especially due to reported muscle toxicity [9] which often translates into reduced therapeutic adherence [10]. In case that statin therapy is not tolerated or does not allow to achieve the recommended LDL-C values, the use of other lipid-lowering drugs is necessary [11]. Additional therapeutical options currently available include ezetimibe and the proprotein convertase subtilisin/kexin type 9 (PCSK9i) inhibitors [12]. The higher cost of this latest strategy makes its wide-ranging use difficult, thus, in several country eligible criteria have been defined [13]. For this reason, scientific research in the field of CVD and especially in the management of atherosclerotic diseases, has led to the development of new therapeutic options to lower LDL-C. Among the emerging molecules, bempedoic acid might represent an interesting emerging option [14]. It is a prodrug activated at the hepatic level, which blocks the synthesis of cholesterol upstream of the target enzyme of statins [15]. The efficacy of bempedoic acid in reducing plasma LDL-C levels has been extensively proven in preclinical and clinical studies [16]. Bempedoic acid faces the complex scenario of dyslipidemia with an intriguing pharmacological potential that could make it an interesting alternative in the management of LDL-C-related atherosclerotic diseases.
2. Bempedoic Acid: Biochemical and Pharmacological Features
2.1. Cholesterol Biosynthesis: The Upstream Effect
Bempedoic acid (2,2,14,14-tetramethyl-8-hydroxy-pentadecanoic acid) belongs to a new class of drugs whose primary mechanism of action is direct, competitive inhibition of hepatic adenosine-triphosphate citrate lyase ACLY [17]. It is a cytosolic enzyme involved in the process of cholesterol synthesis that acts upstream of the enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), target of statins. It is a prodrug that requires an activation step to exert its inhibitory effect, catalyzed by the enzyme very long chain acyl CoA synthetase-1 (ACSVL1), expressed in the liver but absent in the adipose tissue, intestine, and skeletal muscle [15][18]. Hence, bempedoic acid is activated only in the liver without muscle involvement [19]. This allows a reduction in the risk of potential adverse events affecting the muscles, such as myalgia and myopathy, which instead might occur during the statins use [20]. The ACSVL1 enzyme inserts an acyl-CoA into the prodrug [15][18], leading to the formation of the active metabolite, bempedoic acid-CoA, and its reversible conversion to ESP15228, another active metabolite, obtained by oxidation of bempedoic acid [17][21]. ESP15228 probably contributes less to the overall clinical activity than bempedoic acid [21]. The bempedoic acid-CoA complex competes for the ACLY enzyme with the citrate deriving from the mitochondrial Krebs cycle, preventing its transformation into acyl-CoA and thus blocking the chain of biochemical reactions occurring in the synthesis process of cholesterol [15]. This results in a reduction in the endogenous synthesis of cholesterol in the liver [18]. At the molecular level, the block of cholesterol synthesis activates a specific transcription factor (SREBP2) which in turn promotes the transcription of the gene for the LDL receptor (LDL-R), thus leading to an increased expression of the LDL-R [15]. Clinical data document a reduction in LDL-C levels, but also in non-high-density lipoproteins cholesterol (non HDL-C), apolipoprotein B (ApoB), and total cholesterol (TC) in patients with hypercholesterolemia or mixed dyslipidemia [16]. A schematic view of bempedoic acid function in cholesterol biosynthesis is shown in Figure 1.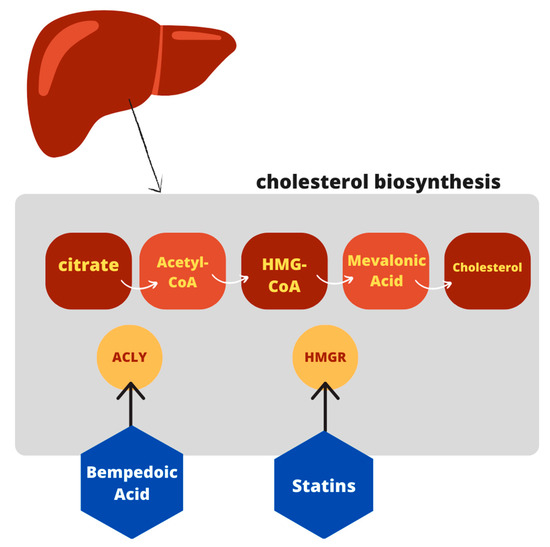
Figure 1. Schematic view of bempedoic acid function in cholesterol pathway.
2.2. Alternative Biochemical Pathway beyond Cholesterol Synthesis
An additional target of bempedoic acid has also been demonstrated in experimental animals: the drug has shown the ability to activate adenosine monophosphate-activated protein kinase (AMPK) [24], which leads to a reduction in plasma cholesterol concentrations, triglycerides, and inflammatory markers. Moreover, the activation of the kinase could have positive effects on glycemia and insulin resistance [25]. However, it appears to be specific for the AMPK beta 1 isoform, which represents the isoform expressed in the murine liver; vice versa in the human liver where the prevalent form is Beta 2, toward which the drug does not show activity [26]. Thus, this hypothesis need to be corroborated by further evidence in humans. A schematic view is provided in Figure 2.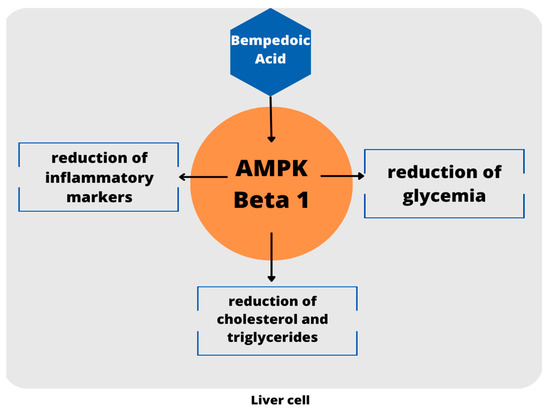
Figure 2.
Schematic view of AMPK activation. To date, these evidence are limited to the mouse model.
2.3. Pharmacological Properties
Bempedoic acid is a drug that is taken orally and absorbed in the small intestine. Concomitant food intake had no effect on the oral bioavailability of the drug. The apparent volume of distribution (V/F) is approximately 18 liters, suggesting a distribution predominantly into the vascular compartment. Bempedoic acid and its active metabolite bind plasma proteins up to 99% [15]. The conversion of bempedoic acid to active drug occurs in the liver. In vitro studies suggest that bempedoic acid, its active metabolite, and glucuronidated forms are not metabolized by cytochrome P450 enzymes thus, indicating few potential interactions with drugs metabolized by cytochrome P450 itself [15]. The active form of bempedoic acid are metabolized to their inactive glucuronide conjugates by the glucuronyltransferase UGT2B7, which is predominantly expressed in the liver and gastrointestinal tract [27]. Once glucuronidated, the two inactive metabolites are mainly eliminated by the kidneys (70%) and partly by the liver (30%) [15]. Bempedoic acid and its metabolites are weak inhibitors of hepatic transporter proteins such as organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3) involved in the hepatic uptake of numerous drugs including some statins such as atorvastatin, prevastatin, fluvastatin, pitavastatin, rosuvastatin, and simvastatin [28]. For this reason, the interactions between bempedoic acid 180 mg and simvastatin 40 mg, atorvastatin 80 mg, pravastatin 80 mg, and rosuvastatin 40 mg have been analyzed in various clinical studies [29]. A study on the combination between bempedoic acid 180 mg plus atorvastatin 80 mg did not lead to a clinically significant increase in the concentration of the latter, so it is possible to administer them in combination [30]. Conversely, the use of bempedoic acid in combination with high doses of simvastatin has shown an increased risk of interactions [15]: in particular, the administration of a single dose of simvastatin 40 mg with bempedoic acid 180 mg at steady state resulted in a two-fold increase in simvastatin acid exposure [29]. It follows that the dose of simvastatin should not exceed 20 mg or 40 mg per day for patients with severe hypercholesterolemia [29]. Bempedoic acid is also a weak inhibitor of the carrier protein organic anion transporter-2 (OAT2) [28], localized at the level of the basolateral membrane of the proximal renal tubular cells and involved in the Na-independent transport of organic anions/dicarboxylates, including uric acid (UA), from peripheral blood to the cytosol of renal cells [31]. This effect could explain the increase in serum uric acid levels following the intake of bempedoic acid. Therefore, monitoring of uric acid levels during the treatment with bempedoic acid [32] is recommended in patients diagnosed with gout [33]. The increased plasma UA levels are transient and reversible on discontinuation of the bempedoic acid treatment [34].References
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297.
- Abdullah, S.M.; Defina, L.F.; Leonard, D.; Barlow, C.E.; Radford, N.B.; Willis, B.L.; Rohatgi, A.; McGuire, D.K.; de Lemos, J.A.; Grundy, S.M.; et al. Long-Term Association of Low-Density Lipoprotein Cholesterol with Cardiovascular Mortality in Individuals at Low 10-Year Risk of Atherosclerotic Cardiovascular Disease. Circulation 2018, 138, 2315–2325.
- Cannon, C.P. Low-Density Lipoprotein Cholesterol: Lower Is Totally Better. J. Am. Coll. Cardiol. 2020, 75, 2119–2121.
- Packard, C.; Chapman, M.J.; Sibartie, M.; Laufs, U.; Masana, L. Intensive low-density lipoprotein cholesterol lowering in cardiovascular disease prevention: Opportunities and challenges. Heart 2021, 107, 1369–1375.
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188.
- Dyrbus, K.; Gasior, M.; Penson, P.E.; Banach, M. Extreme cardiovascular risk-do we need a new risk category? Eur. Heart J. 2022, 43, 1784–1786.
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561.
- Iwaki, Y.; Lee, W.; Sugiyama, Y. Comparative and quantitative assessment on statin efficacy and safety: Insights into inter-statin and inter-individual variability via dose- and exposure-response relationships. Expert Opin. Drug Metab. Toxicol. 2019, 15, 897–911.
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350.
- Birtcher, K. When compliance is an issue-how to enhance statin adherence and address adverse effects. Curr. Atheroscler. Rep. 2015, 17, 471.
- Abdul-Rahman, T.; Bukhari, S.M.A.; Herrera, E.C.; Awuah, W.A.; Lawrence, J.; de Andrade, H.; Patel, N.; Shah, R.; Shaikh, R.; Capriles, C.A.A.; et al. Lipid Lowering Therapy: An Era Beyond Statins. Curr. Probl. Cardiol. 2022, 47, 101342.
- Ciccarelli, G.; D’Elia, S.; De Paulis, M.; Golino, P.; Cimmino, G. Lipid Target in Very High-Risk Cardiovascular Patients: Lesson from PCSK9 Monoclonal Antibodies. Diseases 2018, 6, 22.
- Azari, S.; Rezapour, A.; Omidi, N.; Alipour, V.; Behzadifar, M.; Safari, H.; Tajdini, M.; Bragazzi, N.L. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: A systematic review. Heart Fail. Rev. 2020, 25, 1077–1088.
- Smith, W.; Cheng-Lai, A.; Nawarskas, J. Bempedoic Acid: A New Avenue for the Treatment of Dyslipidemia. Cardiol. Rev. 2021, 29, 274–280.
- Masana Marin, L.; Plana Gil, N. Bempedoic acid. Mechanism of action and pharmacokinetic and pharmacodynamic properties. Clin. Investig. Arterioscler. 2021, 33, 53–57.
- Di Minno, A.; Lupoli, R.; Calcaterra, I.; Poggio, P.; Forte, F.; Spadarella, G.; Ambrosino, P.; Iannuzzo, G.; Di Minno, M.N.D. Efficacy and Safety of Bempedoic Acid in Patients with Hypercholesterolemia: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e016262.
- Ruscica, M.; Banach, M.; Sahebkar, A.; Corsini, A.; Sirtori, C.R. ETC-1002 (Bempedoic acid) for the management of hyperlipidemia: From preclinical studies to phase 3 trials. Expert Opin. Pharmacother. 2019, 20, 791–803.
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Birch, C.M.; Smith, B.K.; Filippov, S.; Groot, P.H.E.; et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 2016, 7, 13457.
- Watkins, P.A. Very-long-chain acyl-CoA synthetases. J. Biol. Chem. 2008, 283, 1773–1777.
- Ruscica, M.; Sirtori, C.R.; Carugo, S.; Banach, M.; Corsini, A. Bempedoic Acid: For Whom and When. Curr. Atheroscler. Rep. 2022, 24, 791–801.
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020, 77, 101006.
- Oniciu, D.C.; Myers, J.L. Bempedoic Acid and the Fraudulent Fatty Acid Family: The Gold Rush to Cardiovascular Therapies in the New Millennium. Org. Process Res. Dev. 2021, 25, 365–372.
- Velazquez, A.M.; Bentanachs, R.; Sala-Vila, A.; Lazaro, I.; Rodriguez-Morato, J.; Sanchez, R.M.; Laguna, J.C.; Roglans, N.; Alegret, M. KHK, PNPLA3 and PPAR as Novel Targets for the Anti-Steatotic Action of Bempedoic Acid. Biomedicines 2022, 10, 1517.
- Paton, D.M. Bempedoic acid. ATP-citrate lyase inhibitor, AMPK activator, Treatment of hypercholesterolemia. Drugs Future 2017, 42, 201.
- Ballantyne, C.M.; Davidson, M.H.; Macdougall, D.E.; Bays, H.E.; Dicarlo, L.A.; Rosenberg, N.L.; Margulies, J.; Newton, R.S. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: Results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J. Am. Coll. Cardiol. 2013, 62, 1154–1162.
- Pinkosky, S.L.; Filippov, S.; Srivastava, R.A.; Hanselman, J.C.; Bradshaw, C.D.; Hurley, T.R.; Cramer, C.T.; Spahr, M.A.; Brant, A.F.; Houghton, J.L.; et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 2013, 54, 134–151.
- Mackenzie, P.I.; Miners, J.O.; McKinnon, R.A. Polymorphisms in UDP glucuronosyltransferase genes: Functional consequences and clinical relevance. Clin. Chem. Lab. Med. 2000, 38, 889–892.
- Biolo, G.; Vinci, P.; Mangogna, A.; Landolfo, M.; Schincariol, P.; Fiotti, N.; Mearelli, F.; Di Girolamo, F.G. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front. Cardiovasc. Med. 2022, 9.
- Jadhav, S.B.; Crass, R.L.; Chapel, S.; Kerschnitzki, M.; Sasiela, W.J.; Emery, M.G.; Amore, B.M.; Barrett, P.H.R.; Watts, G.F.; Catapano, A.L. Pharmacodynamic effect of bempedoic acid and statin combinations: Predictions from a dose-response model. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 578–586.
- Lalwani, N.D.; Hanselman, J.C.; MacDougall, D.E.; Sterling, L.R.; Cramer, C.T. Complementary low-density lipoprotein-cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC-1002) to high-dose atorvastatin background therapy in hypercholesterolemic patients: A randomized placebo-controlled trial. J. Clin. Lipidol. 2019, 13, 568–579.
- Sato, M.; Mamada, H.; Anzai, N.; Shirasaka, Y.; Nakanishi, T.; Tamai, I. Renal secretion of uric acid by organic anion transporter 2 (OAT2/SLC22A7) in human. Biol. Pharm. Bull. 2010, 33, 498–503.
- Ray, K.K.; Bakris, G.L.; Banach, M.; Catapano, A.; Duell, P.B.; Mancini, G.B.J.; Bloedon, L.; Feng, A.; Gotto Jr, A.M. Effect of bempedoic acid on uric acid and gout in 3621 patients with hypercholesterolemia: Pooled analyses from phase 3 trials. Eur. Heart J. 2020, 41, ehaa946-3001.
- Cicero, A.F.G.; Pontremoli, R.; Fogacci, F.; Viazzi, F.; Borghi, C. Effect of Bempedoic Acid on Serum Uric Acid and Related Outcomes: A Systematic Review and Meta-analysis of the available Phase 2 and Phase 3 Clinical Studies. Drug Saf. 2020, 43, 727–736.
- Ballantyne, C.M.; Bays, H.; Catapano, A.L.; Goldberg, A.; Ray, K.K.; Saseen, J.J. Role of Bempedoic Acid in Clinical Practice. Cardiovasc. Drugs Ther. 2021, 35, 853–864.
More
