Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Betty Revon Liu and Version 2 by Catherine Yang.
The peptide-based gene delivery system, mediated by cell-penetrating peptides (CPPs), has been regarded as a promising non-viral tool for efficient and stable gene transfection into both animal and plant cells. CPPs are short peptides with diverse sequences and functionalities, capable of agitating plasma membrane and entering cells. Various basic, amphipathic, cyclic, and branched CPPs were designed, and modifications of functional groups were performed to enhance DNA interaction and stabilization in transgenesis.
- cell-penetrating peptides
- transgenic plants
- gene delivery
- direct membrane translocation
1. Introduction
The current trends in crop yield fall short of meeting the demand, as the global requirement for food is projected to double in the next 30 years [1]. Modern agriculture is facing major global challenges, such as loss of biodiversity, chemical contamination of soils, plant pests, and diseases [2], all of which can directly affect plant health and productivity. Genetically modified plants and crops provide one of the solutions to increase global food production with improved gains in yield and resistance to plant diseases or insect pests. Several successful cases of genetically modified plants have conferred phytoprotection against insects, pests, and pathogens, such as overexpression of proteinase inhibitor genes from legumes [3], recombinant Bt toxic proteins from soil bacteria Bacillus thuringiensis [4], α-amylase inhibitors, and plant lectins [5]. In spite of these successful examples, there is a need to develop alternative strategies of phytoprotection.
Transgenic plants are defined as plants containing gene modifications and expressing recombinant proteins or products from foreign genes [6]. The success of transgenic plants depend on favorable methods of gene delivery. The first transgenic plant was reported in 1983 when an antibiotic-resistant Ti plasmid was delivered into tobacco, mediated by A. tumefaciens [7]. Subsequently, tremendous gene delivery strategies, such as particle bombardment (biolistics), were applied in plants. Flourishing developments of biotechnology in exogenous nucleic acid delivery have brought a great improvement in transgenic plants [8][9][8,9].
Genetic transformation methods in plants were generally divided into two types: direct and indirect gene delivery methods [9]. DNAs or RNAs can be introduced into plants either directly or packaged by specific viruses or bacteria, then transferred into plants via an indirect method [9]. Additional gene delivery classifications include physical, chemical, and biological methods (Figure 1) [10]. Physical methods, such as microinjection, biolistics, electroporation, silicon carbide fibers, laser-mediated DNA delivery, sonoporation, hydrodynamic force, etc., facilitate nucleic acids to penetrate cell membrane directly [11]. The advantages of physical methods for gene delivery are plant- and genome-type independence. Large- or small-sized plasmid DNAs can be delivered by these methods, while DNA transformations in some recalcitrant plants, such as cereals and legumes, are wildly applied [12]. However, the criticisms of physical methods are irreversible tissue damage and integration of genes into host genomes [10]. In microinjection and electroporation methods, plant sample preparations become protoplasts or a single cell, and this complicated procedure makes the drawback for transgenic plant applications [10].
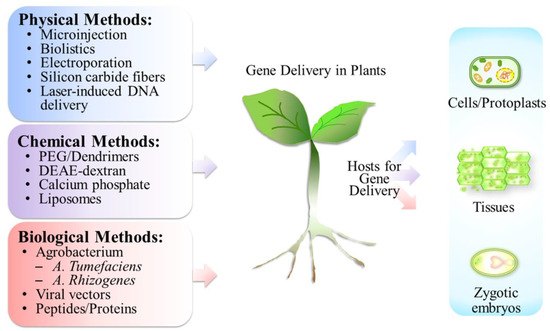
Figure 1. Methodologies of gene delivery in plants. Various methods including physical, chemical, and biological manners were applied in gene delivery. Plant cells were prepared as protoplasts for gene uptake. Plant tissues (callus) and zygotic embryos also served as the transgenic hosts. PEG: polyethylene glycol; DEAE: diethylaminoethanol.
Polyethylene glycol (PEG)-, diethylaminoethanol (DEAE)-dextran-, calcium phosphate-, dendrimer-, and liposome-mediated gene transfers were categorized as chemical methods [11][13][11,13]. Hygromycin resistance gene was introduced into the protoplasts of rice, and these transgenic rice plants generated viable seeds [14]. The benefits of liposome-mediated nucleic acids delivery include DNA protection from nuclease digestion, as well as suitability for multiple types of plant cells, such as protoplasts and plasmodesmata [15]. Calcium phosphate is a cheap and easily handled DNA delivery method that possesses economic benefits. DNAs are able to interact with positively charged calcium ions via electrostatics, form precipitates, and enter cells by endocytosis [11]. Endocytosis is the major route for DNA delivery in chemical method-mediated plant transformation [16]. However, nucleic acids, which might be digested in lysosomes, and the limitation on plasmid DNA size reduce the potential of application in transgenic plants [16][17][16,17].
2. Cell-Penetrating Peptides (CPPs)
CPPs are short and membrane-active peptides [18][21]. In general, macromolecules, such as DNAs, RNAs, and proteins, are impermeable to cell membranes. The cell membrane is a natural barrier to prevent harmful exogenous or pathological molecules from entering the cell freely, and to maintain the osmotic balance within the cell. Some functional proteins are able to enter cells via specific receptors or channels, while nucleic acids alone are generally not [19][23]. Not only can CPPs enter cells by themselves, but also deliver various cargoes, including nucleic acids, into living cells [20][24]. However, nucleic acids are not the only macromolecules that CPPs are able to deliver. CPPs also can serve as a Trojan horse, while peptides/proteins [21][25], nanoparticles [22][26], pharmaceutical molecules, and small drugs [20][23][24][24,27,28] play the role of Achilles. The selectivity and efficiency of drug/molecules delivery are significantly improved when CPPs cooperate with liposomes/micelles [25][26][29,30]. Most CPPs have been shown to be nontoxic, and do not interfere with functionality of the delivered biomacromolecule [27][31].
There are various interactions between CPPs and their cargoes. CPPs and cargoes can form complexes with covalent bonds [28][32], noncovalent interactions [18][21], and covalent- and noncovalent-synchronous linkages [29][33]. The potential of bio-membrane penetration in CPPs is amazing. Until now, studies indicated that CPPs were able to penetrate different targets, including mammalian cells [18][21], plant cells/tissues [28][29][30][31][32][33][34][35][32,33,34,35,36,37,38,39], rodent skin and intestinal mucosa [21][25], prokaryotes [36][37][40,41], fungi [38][42], insect cells [39][43], paramecia [40][44], and rotifers which were individual organisms containing thick cuticles [41][45]. Recently, different modifications on CPPs, such as D-form amino acid applications, branches on backbone sequences, cyclic structures alterations, and non-standard amino acid substitutions were designed to increase their internalization efficiency and stability [18][42][43][44][21,46,47,48]. Highly cellular penetration efficiencies and non-cytotoxic properties make CPPs an ideal delivery system for therapeutic drugs, gene therapies, and transgenic plants [21][45][46][47][48][22,25,49,50,51].
3. Subcellular Targets for Gene Delivery
CPPs have demonstrated remarkable ability to deliver diverse biomacromolecules into various plant species. The plasmid DNA delivery mediated by CPPs displayed a high potential and efficiency in plant root cells [49][67], embryos [50][97], and leaf cells [51][82] without protoplast preparations. Positively charged CPPs possess the abilities to interact with, condense, and package plasmid DNAs. The combination ratio between CPP and nucleic acid (Figure 2), also called nitrogen (NH3+)/phosphate (PO4−) (N/P) ratio [52][98], is key to DNA condensation and packaging. It further affects gene delivery efficiency [49][53][53,67]. An optimal N/P ratio makes CPP/DNA complexes more stable and is able to raise gene delivery efficiency. A good transgenic efficiency also depends on other factors, such as long-term stability of CPP/DNA complexes in cytosol, evasion from the endosome–lysosome system, targeted site of gene expression, and DNA releasing from CPP/DNA complexes [30][34][54][55][56][34,38,81,83,99]. The efficiency of cytoplasmic delivery by the predominant endosomal pathway is typically very low. A study showed that glutathione-responsive CPPs are able to escape from endosome entrapment and release DNAs at a higher rate to achieve gene transfer in plants [30][34]. Aside from efficiency, targeted delivery is also crucial in transgenic plant development [57][100]. Various DNA plasmids were designed and applied to the genes that were successfully achieved for development of transgenic plants (Table 12). ThHe researchersre, we discussed three major subcellular targets for CPP/DNA complex delivery: nucleus, plastids, and mitochondria (Figure 2).Table 12.
List of various genes applied in transgenic plants.
| Delivery Methods | Genes | Targets | References |
|---|---|---|---|
| Non-CPP-based gene delivery | Proteinase inhibitor genes | Tobacco | [3] |
| Recombinant Bt toxic proteins | Vigna ungiguiculata | [4][5][4,5] | |
| α-Amylase inhibitors, plant lectins | Adzuki bean | [5] | |
| Antibiotic-resistant Ti plasmid (A. tumefaciens mediated transfection) |
Tobacco | [7] | |
| Hygromycin resistance gene | Protoplasts of rice | [14] | |
| CPP-based gene delivery | p35S-RLuc-tNOS and p35S-GFP-tNOS plasmids | Leaves of A. thaliana | [30][34] |
| p35S-Nluc-tNOS or p35S-GFP-tNOS plasmid | Seedlings of A. thaliana | [34][38] | |
| pHBT-sGFP(S65T)-NOS plasmid | Roots of mung bean and soybean | [49][67] | |
| psbAp:GFP:SPECr:psbAt At plastid genome integration vector, cox2p: GFP:SPECr:cox2t At mitochondrial genome integration vector, and cox2t:SPECr:GFP:cox2p Nt mitochondrial genome integration vector | Seedlings and leaves of A. thaliana or N. tabacum | [58][78] | |
| PsbA-SPECr-sGFP-psbA, Prrn-aadA-sfGFP-Trps, PsbA-SPECr-sGFP-psbA, and Prrn-aadA-sfGFP-Trps | Leaves of A. thaliana | [59][79] | |
| pDONR-cox2:rluc and pDONR-cox2:gfp plasmids | Leaves of A. thaliana | [54][81] | |
| pAct-1GUS plasmid | Wheat immature embryos | [50][97] | |
| pPrrn::GFP(S65T)::TpsbA, pPrrn::DsRed::TpsbA, and pPpsbA::Rluc plasmids | Leaves of A. thaliana | [56][99] | |
| psfGN155-MxMT and psfGC155-MxMT plasmids | Leaves of N. benthamiana | [57][100] | |
| pBI221, pBI121, and pPpsbA::Rluc plasmids | Leaves of Arabidopsis, soybean, and tomato | [60][101] |
