Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tariq Aziz and Version 2 by Jessie Wu.
Microorganisms can produce nanoparticles through both intracellular and intercellular biosynthesis. This process can occur in a variety of different microorganisms, making it a versatile method for nanoparticle production. Different types of microbes can control the synthesis of various metallic nanoparticles.
- nanotherapeutics
- nasal
- tissue
1. Introduction
The nose has great importance and is positioned under and in the middle of the eyes. The nostrils open into the esophagus and trachea and are the back entrance. Oppositely nares have exterior openings. The nose is the main and most important opening of the respiratory tract. It plays a basic role in respiration. It performs many functions such as warming, moisturizing, and filtering air when it passes through the nose. It also plays an important role due to the presence of olfactory organs [1][89].
2. Nasal System
The nasal cavity has a typical shape, and it extends from the bony plate to a pointing cranium. The bony plate is made up of seven bones. Due to this system, a chamber is created which is 7.5 cm and 5 cm in length and width. The nose is separated into two parts with the help of the nasal septum [2][90]. The vomer is also present in it and is located anteriorly to the ethmoid bone and posteriorly to the septal cartilage. The nasal septum is extended from the nares to the nasopharynx. A soft plate, hard palatine bone, and tissue flap form the roof of the mouth and floor of the nose. The soft plate creates a system to avoid being stuck in food at the nose back. After food passage, the soft plate moves upward and clears the way for air. This makes the capacity to breathe from the nose as well as from the mouth but breathing has some advantages such as humidity, filtration, and heating of the air [3][4][91,92].
The nasal cavity is known for transferring infection, due to its larger site for entry of the pathogens into the body without resistance. This site of the body possesses a peak permeability factor with less enzymatic activity. The microvillus region of the nose allows the capacity for vaccine particles to be absorbed and digested through the nasal cell wall. The first line of defense is humoral immunity involved in the production of B-cells by immunoglobulin A (IgA), which is available in the nasal cavity in a dimeric shape; the defense cells of lymphoid tissues are used in the activation of cellular and humoral immunity in the body. The main achievement of vaccination is to keep B and T cells active against antigen particles [5][93].
The components involved in the manufacturing of nanoparticles are polymers including polylactide and polyglycolide. Few other particles such as copolymers and polyacrylates with natural chemicals such as gelatin and collagens are included. These polymers have been used for animal and human health. A form of modulating polymers can be used for therapeutic capacity of nanoparticle application to humans up to the desired level.
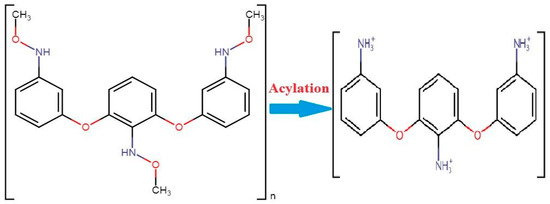
Another polymer, polyethylene glycol, is important against corona as a nasal protein. The stable character of this chemical makes it more attractive for nasal application while escaping from enzyme interactions. Chitosan polymer creates a barrier between antigen and body humoral immunity while inhibiting the escape of antigens from body immunity. A mixture of chitosan enhances the absorption power of the nasal cavity. These techniques may be helpful in the application of vaccines. Researcher De-Haan and his colleagues have successfully delivered a vaccine using liposomes [6][94]. They proved the efficacy of using of liposomes against the influenza virus with vaccine subunits. An experimental application of chitosan was performed against hepatitis B. This viral disease kills more than 25% of the world’s population every year. A vaccine is being produced against it, but it are manually applied, which also causes pain so the administration is not suitable. For that reason, nanoparticle applications have been introduced to treat life threatening diseases. When chitin combines with acylation it results in chitosan. These particles have a size of 250 nm to 350 nm with more than a 28,000 molecular weight. A dry form of the chemical tripolyphosphate has been used in the purification of chitosan manufacturing during the acylation of chitin (Figure 1) [7][95].
A study shows that the nanoscale method of vaccine delivery is more efficient and effective than oral uptake of medications. A huge number of novel therapeutic chemicals, including proteins, plasmid DNA and peptides have been developed. Nasal medications are facilitated by (NALT) nasal-associated lymphoid tissue, which holds aerosols of the vaccine within its epithelial surface [8][96]. The oral intake of medicine or chemicals is a little complicated by low chemical absorption rate, less bioavailability, low enzymatic breakdown, and some other substituent effects on the digestive system of the body. This draws the focus of chemical industries and pharmaceutical companies toward nasal ingestion of medicine. For nasal inhalation of medicine, a low molecular weight of medicine is required, especially at that time when rapid action of the medicine is required [9][97].
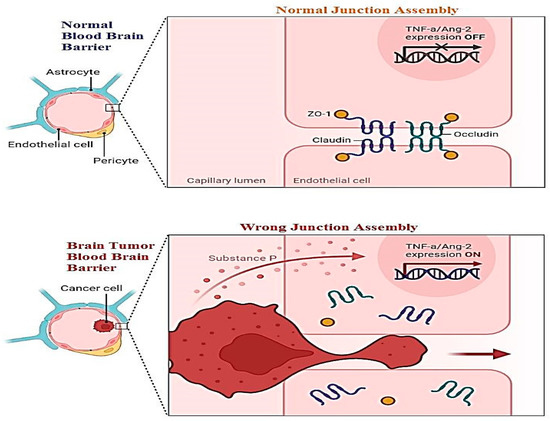
A new challenge must be health-related staff to manage the blood–brain barrier. This layer relates to all the parts of the brain including blood capillaries. Direct medication from the nose to the brain is a novel methodology of treatment. For this purpose, two pathways have been introduced: the first is trigeminal, and the second is an olfactory passageway for treatment with an application of nanoparticles. Drawbacks of these techniques including cell damage and the inflammatory response have been observed. Nanoparticles are best used for the delivery of DNA vaccines. Vectors used for the transportation of DNA vaccines are lentiviral vectors and adeno-associated-viral-vectors (AAVV). An alternative to viral vectors are nanoparticles highlighted for the safe delivery of DNA vaccines. Figure 2 shows the difference between the normal blood–brain barrier and the brain tumor blood–brain barrier. It shows swelling and clotting of blood cells inside the layer which resist cerebrospinal fluid (CSF) transport of material across the wall [10][98].
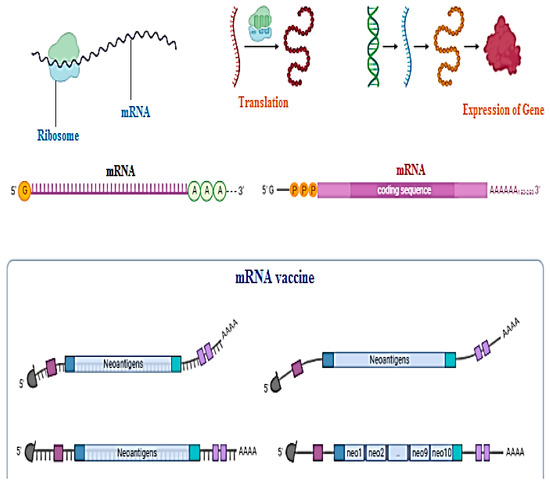
Messenger RNA or DNA vaccine techniques are used to make clones of antigens just to trigger the body’s immune response to become immunized against disease [11][12][13][14][99,100,101,102]. Not a single DNA vaccine has been approved by the FDA, especially for human use, but in the future, they could be utilized for the service of mankind. Nanoparticles are a hot issue for application of DNA vaccines today. The beneficial aspects of DNA vaccines as compared to traditional vaccines are that they create no issues with recombinant proteins such as wrong folding of proteins or becoming reduced during structural formation, its low cost of synthesis, and that it can trigger both lines of immunity, cellular as well as humoral. Chemical changes in the coding gene region, poly-A-tail, can provide an edge to structural stability and authenticity of DNA vaccines. DNA vaccines need to enter the nucleic acid region for their activation as compared to mRNA vaccines for which the cytosol area is enough for activation as shown in Figure 3 [14][15][102,103].
3. Zx+10a0 Nasal Vaccination: Why Nanoparticles?
4. Zx+10a0 Nasal Vaccination: Why Nanoparticles?
In present times, the nasal route is gaining growing interest and some drugs having low molecular weight have been approved and are available in the market. Butorphanol is used for pain relief and is applied via the nasal route. For cryptorchidism, luteinizing hormone-releasing hormone (LHRH) is used and is taken via the nasal route. Many other drugs having low molecular weight are also being applied via the nasal route [15][103]. In the case of larger molecules, (for example, proteins) this route is not used frequently. This is due to the drug absorption problem. Hence, further strategies should be developed to improve drug absorption [17][104]. Figure 4 shows the inhalation of a vaccine through the nasal cavity and its mechanism inside the body in immune cells.
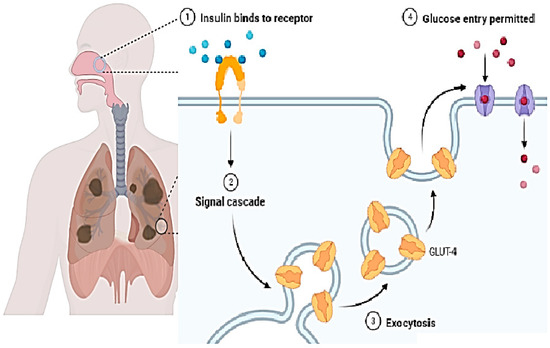
The mass cut-off value for the permeation of molecules via the nasal system is approximately 1000 Da. Hence, larger molecules are not allowed, and absorption enhancers are required for this. To overcome the problem, nanoparticles are the best options as they are successful adjuvants that are used as the delivery system or/and immune modulators for the application of vaccines [18][105]. They also have a beautiful attribute in that they can protect against antigenic degradation from proteolytic enzymes, hence ensuring drug delivery at the cellular level. An interesting fact is that they can pass the mucus and directly interact with the mucosal cells and enhance immunity activation. One can also change the attributes of the nanoparticles to obtain the results of one’s own interest. Due to their unique small size, they also show mimicry with viruses [19][106]. Due to their small size, they can pass through the mucus barrier. Therefore, they have high cellular interaction. The size of nanoparticles ranges from approximately 20 nm to 80 nm. This wide range is administered in a dose making them interesting to use. They also have a hydrophobic nature which helps them pass the mucosa. These important features of nanoparticles make them a strong candidate for use in drug delivery via the nasal cavity [20][107].
4. Features of the Nose for the Delivery of Drugs and Vaccines
5. Features of the Nose for the Delivery of Drugs and Vaccines
To understand the phenomenon of immune system triggering during the delivery of a drug, it is important to compare rodent with human noses. In the case of rodents, nose-associated lymphoid tissues (NALT) are lymphoid tissues [21][108]. They are present at the dorsal nose duct bottom. These are bell-shaped and paired tissues and lymphoid tissues are accumulated in their formation. This process is completed in around 5–8 weeks after birth. In human beings, NALT is composed of tonsils and adenoids. This is an important feature of the human mucosal immune system. “Waldeyer’s ring” was identified in 1884 by Waldeyer. It is formed of adenoid or nasopharyngeal tonsils in addition to “paired palatine tonsils”, “the paired tubal tonsils” and lingual tonsils [22][109].
Tonsils are in the lamina propria of the pharyngeal wall, and these are secondary lymphoid organs. When observed under a microscope, the surface of the tonsils is made up of various epithelial linings and are called crypts. These crypts deeply penetrate the lymphoid tissues. They play an important role in the respiratory immune system by increasing the surface area of the tonsils [23][110]. They are specially designed to trap foreign materials. The histology and anatomy of both human and mouse nasal cavities are different. In the case of mice, single-layer epithelium makes up the murine respiratory epithelium. Along with this, in the turbine portion, columnar epithelial cells are present. The olfactory epithelium of mice is covered with “pseudostratified columnar epithelium”. In the case of human beings, there is no single epithelium layer. Pseudostratified columnar epithelium covers both the olfactory and respiratory surfaces. Notably, in nasal epithelium cells and the upper airway, tight junction (TJ) molecules are present. Due to the presence of these structures, permeability of the human nasal epithelium layer is poor [24][111].
Activation of the Mucosal Immune System by Nanoparticles
There are two main components of the mucosal immune system keeping in view the anatomy and structure. These two components are [25][26][112,113]:
- (1)
-
effectors sites;
- (2)
-
inductive sites.
In inductive sites, an antigen-specific immune system is activated, and it is composed of “organized mucosa-associated lymph tissues”. The effector sites are usually associated with “cell-mediated response” and with the production of antibodies [27][114]. NALT is a site of both humoral and cell-mediated activation. In NALT all those cells that are necessary for immune response are present. Here B cells, T cells, dendritic cells, and antigen-presenting cells (APC) are present. All these sites are directly associated with the inductive sites through the mucosal immune system for antigen-specific immune response. This system works as the first line of defense at the mucosal surface [28][115].
M cells are also present in NALT. Their basic work is to screen antigens to recruit potential pathogens for immune response. M cells perform two very important functions [29][116]:
- (1)
-
barrier maintenance;
- (2)
-
mucosal immune response initiation.
M cells in NALT are the key players in nanoparticle uptake and producing IgA antibodies. Mucus is the epithelial layer and is the first barrier that nanoparticles must face. The epithelial layer not only acts as a barrier line but it also secretes cytokine and activates an innate immune response [30][117].
The adaptive immune response is initiated by the APC with the help of MHC. Dendritic cells (DC) can move from NALT to lymph nodes and stimulate the helper T cells or cytotoxic T-cell response. In this process, with the involvement of NK cells and eosinophils at the end, the cell is lysed [31][118].
5. Nasal Drug and Vaccine Delivery and Nanoparticles
6. Nasal Drug and Vaccine Delivery and Nanoparticles
There are four major types of nanoparticles that have been prepared and are being used for drug and vaccine delivery via the nasal cavity [25][26][112,113]:
- (1)
-
polysaccharide nanoparticles;
- (2)
-
protein nanoparticles;
- (3)
-
lipid nanoparticles;
- (4)
-
polymers nanoparticles.
Different laboratories around the world use different methods of administration. Some information about the dose, volume, antigen concentration, number of administrations, and or without the process of anesthesia are summarized in Table 1. Next rwesearchers discuss the different types of nanoparticles used in the delivery of drugs and vaccines [32][119].
Table 1.
Nanoparticles for nasal vaccination, immunization parameters, and immune response.
| Type of Particles | Particles Characteristics | Model | Immunization Parameters | Immunity Response |
Reference | |||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Z (mV) | Admi. | Ag Dose (µg) | Anes. | ||||
| Chitosan | 300–680 | 26 | Mice | 3 × 15 µL | 2.5 | Yes | h, m | (Bento et al.) [33][120] |
| 80 | 14 | Mice | 2 × 10 µL | 10 | Yes | h, m | (Pawar et al.) [34][121] | |
| Maltodextrin | 70 | 38 | Mice | 3 × 12 µL | 10 | No | h, c, m | (Debin et al.) [35][122] |
| Chitosan PLGA | 500 nm–2 µm | - | Cattle | 1/2/3 mL | 10–15 | - | h, m | (Pan et al.) [36][123] |
| Liposomes | 30–100 | - | Mice | 3 × 50 µL | - | - | c | (Ninomiya et al.) [37][124] |
| ISCOMs | 40–50 | - | Mice | 2 µL | 2 | Yes | h, m | (Cibulski et al.) [38][125] |
| Polystyrene | 300–390 | - | Mice | 3 × 20 µL | 10 | - | c | (Misstear et al.) [39][126] |
| PLA microparticle | - | - | Mice | 10 × 50 µL | - | Yes | h | (Rice-Ficht et al.) [40][127] |
| Lipopeptide | 150–1000 | - | Mice | 3 × 10 µL | 40 | - | h, m | (Zaman et al.) [41][128] |
| Dextran | 140–310 | −38:39 | Mice | 3 × 10 µL | 10 | No | h, m | (Marasini et al.) [42][129] |
Abbreviations: Zeta potential (Z), Antigen (Ag), Anesthesia (Anes.), Administration (Admi.), the response of antigen is indicated as h—humoral, m—mucosal, c—cellular, Polylactic-co-glycolic acid—PLGA, polylactic acid (PLA).
5.1. Polysaccharide-Based Nanoparticles
6.1. Polysaccharide-Based Nanoparticles
Polysaccharide nanoparticles have been widely used as delivery systems thanks to their important property of “biocompatibility”.
5.2. Chitosan Nanoparticles
6.2. Chitosan Nanoparticles
Chitosan is an important polymer of N-acetylglucosamine and glucosamine. It is derived from the partial deacetylation of chitin. Chitin is abundantly present in shellfish. It has been used as an absorption enhancer in many drugs. It is mucoadhesive and is soluble at acidic pH [33][120]. Its efficacy is dependent upon the degree of deacetylation. The nature of these nanoparticles is mucoadhesive. Due to this property, they have more retention time, and their clearance is delayed.
Therefore, the retention time in the mucosa is also increased [34][121]. This also increases the contact time between formation and NALT. Due to the mucoadhesive nature of chitosan nanoparticles, the residence time of the administered solution could be quadrupled, thanks to this unique property.
They also have adjuvant properties and are very successful in the case of different protein antigens such as recombinant anthrax, influenza, hepatitis B, and ovalbumin. When experiments were conducted on a mice model, they showed an improved version of humoral (IgG) and mucosal (IgA) responses. These nanoparticles were loaded with toxoid of tetanus, and they triggered both IgA and IgG production [35][36][37][38][39][40][41][42][43][44][45][122,123,124,125,126,127,128,129,130,131,132].
5.3. Starch Nanoparticles
6.3. Starch Nanoparticles
Starch is a polysaccharide found in nature, and it is composed of amylopectin and amylose. This carbohydrate is abundant in the amyloplasts of plants. Here it works as an energy reserve. As a result of partial starch hydrolysis, maltodextrin is obtained. It is also used in the synthesis of nanoparticles [46][133]. These are biodegradable and have a wide range of applications in drug delivery and nano vaccines. In an experiment, influenza virus antigens were encapsulated in a propyl acrylic acid and starch mixture. As a result, an IgG-specific response was achieved. In the case of intranasal vaccination, maltodextrin is a promising mucoadhesive polysaccharide nanoparticle. When it was loaded with the antigen of hepatitis B, it showed more mucosal, humoral, and cellular immune responses than did free antigens [47][134].
6. Polymer Nanoparticles
7. Polymer Nanoparticles
Co-polymer of lactic acid and glycolic acid (PLGA) is one of the most important synthetic polymers for nanoparticle preparation. They are biodegradable and biocompatible, thanks to their unique properties. Polylactic acid (PLA) could be used for nanoparticle preparation to delay the rate of delivery for drugs having low molecular weights. Glycolic acid has been added to PLA to formulate PLGA [47][134]. Now, a very wide and unique variety of PLGA polymers is available in the market. Different ratios of moles have been used in them for the formulation of polymers having unique and different properties. They have either an acid or ester as a terminal group, which determines their hydrophobicity. PLGA has the capability of antigen encapsulation in the structure of the matrix, or they can adsorb proteins on the surface. Encapsulation of antigens allows the controlled and sustained release of antigens. This leads to an improved response of the immune system. If PLGA is coated with polyethylene glycol (PEG), it favors the passage of antigens and drugs from the mucosa. Naturally, PLGA is negatively charged but they can be converted to be positively charged by adding cationic ligands (e.g., chitosan). PLGA nanoparticles also play their role in increasing drug permeability into the mucosa. PLGA nanoparticles, when conjugated with transferrin, showed good gene delivery results via the nasal cavity [48][135]. PLGA nanoparticles also have a wide range of applications in veterinary medicine. PLGA particles were loaded with rE2 glycoprotein and showed good results when administered via the nasal cavity as compared to oral administration. So far, most polymer nanoparticle studies have been limited to preclinical development [49][136].
7. Lipid-Based Nanoparticles
8. Lipid-Based Nanoparticles
They have a hybrid structure between liposomes and polymer nano-capsules. Triglycerides of the medium chain form their core which is surrounded by polyethylene glycol (PEG) and lecithin. They can be prepared in a solvent-free environment, which makes them unique. Another important property is their high stability as compared to some other lipid-based nanoparticles [49][136]. In one study, toll-like receptors (TLR) were combined with lipid nano-capsules and prepared for mucosal drug delivery. When applied in mice, it showed long-living T cells in the lungs and vaginal mucosa. These are powerful tools for mucosal drug delivery and vaccination but very little work has been published on this topic so far [50][51][52][137,138,139].
Immune Stimulating Complexes (ISCOMs)
ISCOMs are about nm in size and have an open-caged spherical shape. They are usually made up of phospholipids, cholesterol, and Quil A (usually extracted from the bark of a plant named Quillaja Saponaria). The hydrophobic portion of Quil A is made up of carbohydrates while the hydrophilic portion is made up of quillaic acid, so it is amphiphilic [53][140]. Due to the presence of glucuronic acid at their surface, they are negatively charged. Some cationic derivations of them have also been prepared. Ovalbumin-ISCOMs were prepared, and they induced mucosal and systemic immune responses in mice. In the case of larger animal models, intranasal administration of ISCOMs showed stimulation of IgA production. They showed good results in the case of bovine respiratory syncytial virus. There are very few brands of ISCOMs available in the market for nasal vaccines and drug administration [54][141].
