Biological aging is characterized by irreversible cell cycle blockade, a decreased capacity for tissue regeneration, and an increased risk of age-related diseases and mortality. A variety of genetic and epigenetic factors regulate aging, including the abnormal expression of aging-related genes, increased DNA methylation levels, altered histone modifications, and unbalanced protein translation homeostasis. The epitranscriptome is also closely associated with aging. Aging is regulated by both genetic and epigenetic factors, with significant variability, heterogeneity, and plasticity. Understanding the complex genetic mechanisms of aging will aid the identification of aging-related markers, which may in turn aid the development of effective interventions against this process.
- Genetics
- Aging
- cellular senescence
1. Introduction
2. The Genetics of Aging
The lifespans of different biological species lie within a relatively stable range, and there are significant differences between species, which are indicated in the databases (Database of animal ageing and longevity) as summarized in (Table 1). Multiple factors contribute to this difference, including the ratio between body size and heart rate, environmental factors, energy uptake, and genetic factors [16][9]. In terms of genetic factors, whole genome sequencing has revealed that the mutation rate of non-germline somatic cells between species is an important factor affecting lifespan, with the somatic cell mutation rate having a strong inverse relationship with lifespan and no obvious correlation with body size [17][10]. Another important genetic factor is the telomere, which is a repeating double-stranded fragment located at the end of chromosomes in eukaryotic cells, where it maintains the integrity of chromosomes and contributes to controls cell division cycles [19][11]. Although the relationship between telomere length and species lifespan is somewhat controversial, increasing the length of telomeres in mice has been demonstrated to prolong their lifespan, and telomere shortening rate is an important factor affecting the lifespan of species [20][12]. The genetic basis of longevity is also closely associated with sex, age, and environmental factors, with the influence of genes on lifespan depending on sex, age, and genetic effects varying between males and females [21][13].| GenAge | The ageing gene database | https://genomics.senescence.info/genes/index.html | [28][18] |
| AnAge | Database of animal ageing and longevity | https://genomics.senescence.info/species/index.html | [28][18] |
| CellAge | Database of Cell Senescence Genes | https://genomics.senescence.info/cells/ | [29][19] |
| LongevityMap | Human longevity genetic variants | https://genomics.senescence.info/longevity/ | [30][20] |
| NIA Interventions Testing Program (ITP) Genetics | Conserved longevity gene prioritization | https://www.systems-genetics.org/itp-longevity | [21][13] |
| Aging Atlas | Transcriptomics Epigenomics Single-cell Transcriptomics Proteomics Pharmacogenomics Metabolomics |
https://ngdc.cncb.ac.cn/aging/index | [31][21] |
2.1. Aging-Related Genes and Signaling Pathways
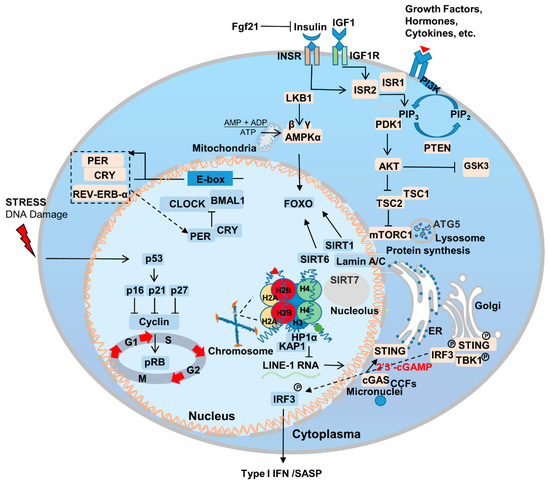
Aging is the most important risk factor for a broad array of diseases, including neurodegenerative diseases, cardiovascular disease, metabolic syndrome, chronic inflammation, and cancer. Additionally, genetic mutations that delay aging have been also found to delay the onset of age-related diseases [36,37][26][27]. Genes that regulate aging are relatively conserved among species and are enriched in certain signaling pathways [6] (Figure 1). The association between aging and disease makes fighting aging an even more attractive proposition; it is likely to fight the occurrence and progression of other diseases as well. In this section, wresearche rs summarize the most important pathways and genes to the aging process.2.2. Nutrient Sensing
Cells rely on nutrient sensing for both the detection of stresses and, ultimately, their survival [38][28]. Nutrient availability and perception are important material basis for maintaining cell growth and normal function, and cellular metabolic homeostasis imbalance and cellular senescence complement each other [39][29]. For example, in Caenorhabditis elegans (C. elegans), mutations in the highly conserved daf-2 gene, which encodes an insulin-like receptor and regulates the insulin/insulin-like growth factor 1 (IGF-1) pathway, have been found to significantly prolong lifespan [40][30]. During aging, the mechanistic target of rapamycin (mTOR) signaling pathway is also important for perceiving stress signals and nutrient sensing, and protein translation [41,42][31][32]. Genetically inhibiting the insulin/IGF and mTOR pathways has also been demonstrated to extend mouse lifespan [43][33]. In 1939, researchers discovered that calorie restriction (CR) can ameliorate aging [44][34]. CR induces various metabolic changes in the body, and crosstalk between CR and proteins related to nutrient sensing-related pathways is an important reason for ameliorate aging [45][35]. To date, CR has been shown to extend the lifespan of Saccharomyces cerevisiae, C. elegans, normal and progeria mouse models, and non-human primate rhesus monkeys; at present, CR represents the most effective lifespan-extending intervention across species [46,47,48,49,50,51,52][36][37][38][39][40][41][42]. This is due to the fact that most molecular pathways involved in longevity are associated with increased stress resistance [53][43]. Compared with ad libitum access to food (AL), every-other-day feeding (EOD) increases the healthy lifespan of mice. Dietary restriction has also been found to limit the growth of various types of tumors [54][44]. The phosphatidylinositol-3-kinase (PI3K) pathway, which is a key insulin signaling component, is an important regulator of CR [50,55][40][45]. In addition, restricting the amount of branched-chain amino acids (BCAAs), such as leucine, in the diet has also been demonstrated to prolong the lifespan of LmnaG609G/G609G and Lmna–/– mice. In terms of physiological aging, a low-BCAA diet reduces weakness, but does not extend lifespan [56][46]. Thus, achieving CR via the regulation of metabolism and diet represents a promising anti-aging intervention.2.3. Sirtuins
Sirtuins are another gene family that can extend the lifespan of C. elegans [57][47]. They are mainly responsible for regulating cell metabolism, genome stability, gene expression, signal transduction, and important for maintaining the health of the body [58][48]. There are seven sirtuins in mice and humans, and, under CR, SIRT1 expression is upregulated. This prolongs lifespan and is closely associated with the IGF signaling pathway [59][49]. Meanwhile, SIRT6 regulates the IGF1 levels and, thus, aging, with SIRT6 overexpression extending lifespan in male mice [27][17]. Recent studies have also confirmed that SIRT1 is a key protein in the regulation of endothelial cell aging; vascular endothelial cells are essential for maintaining the health and growth of blood vessels. Reducing the expression of SIRT1 in endothelial cells accelerates cellular aging and hinders the normal function of blood vessels [60][50]. Endothelial cell senescence performs a pivotal role in systemic aging, but the effects can be lessened via the overexpression of SIRT7 [61,62][51][52]. Furthermore, SIRT6 expression in endothelial cells has been shown to be important for maintaining heart function [63][53]. Taken together, these findings indicate that aging-related genes show tissue-dependent effects, and targeting specific types of senescent cells may represent an effective way to treat systemic aging.2.4. Nuclear Skeleton-Associated Proteins
Intranuclear proteins, such as Lamins play an important role in regulating and maintaining the balance of aging and tumors. Mutations in the LMNA gene affect aging through a number of mechanisms. For example, Lamin A/C interacts with SIRT1, 6, and 7 and affects their intracellular activity and stability, thereby regulating aging [62,64,65][52][54][55]. Interactions between Lamin A/C and SIRT7 also inhibit the transcriptional activation of long interspersed elements-1 (LINE-1, L1) [66][56], which stabilizes heterochromatin structure. This inhibits the development of a SASP, such as a type I interferon response that triggers natural immune pathways, and can therefore delay the aging of human stem cells by reducing inflammation [67][57]. IGF-1/AKT signaling pathway protects cells from apoptosis [68][58]. Furthermore, recent studies have found that the abnormally processed progerin, which is classically located within the nucleus, is also localized outside it. Here, it interacts with IGF-1R and downregulates its expression, thereby impairing IGF-1/AKT signaling, inhibits cellular energy metabolism and accelerates cell aging [69][59]. Inhibiting isoprenylcysteine carboxylmethyltransferase (ICMT)-associated activation of AKT-mTOR signaling has been found to improve progeria symptoms [70][60]. Notably, an mTOR hypomorphic allele (MtorΔ/+) has also been found to improve aging characteristics and lifespan in LMNAG608G mice [71][61]. Taken together, these findings indicate that Lamin A and nutrient sensing share an intricate, important connection to the aging process. Furthermore, another protein from the nuclear matrix, Lamin B1, is also closely related to aging. Cells respond to carcinogenic pressure by degrading Lamin B1 through autophagy, thus accelerating cell senescence [72][62]. Recent studies have found that intranuclear SIRT1 protein is the second major nuclear substrate for LC3-mediated selective autophagy, thus influencing cellular senescence through this degradation mechanism [73][63].2.5. Immunity and Inflammation
Inflammaging is an important component of aging, which is a pathological phenomenon that brings together our knowledge of age-related chronic diseases, functional decline, and weakness [74][64]. In the process of aging, the innate and acquired immune system is remodeled, and the reliability and efficiency of the immune system decrease with age, which leads to the upregulation of inflammatory response and the occurrence of related degenerative diseases [75][65]. The drivers of the inflammatory response mainly include two parts: the degradation of immune receptors/immune sensors and the increase in stimuli that trigger inflammation [36,76][26][66]. Inflammation is also the result of lifelong exposure of the immune system to antigenic stimuli and complex genetic, environmental, and age-related mechanisms. Inflammation underlies aging and many age-related chronic diseases, which in turn increases the rate of aging [36][26]. Excess nutrients are an important factor in inflammation, diet performs an important role in the development and treatment of inflammation and related problems, and CR can slow inflammation and improve aging [77][67]. The activation of innate immune Toll-Like receptors perform an important role in the aging process, and when Toll-like receptors are knocked out, it can significantly ameliorate the aging of heart-related cells [78][68]. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway plays an important role in regulating inflammatory response, and the inhibition of JAK/STAT signaling pathway can reduce age-related inflammatory response to a certain extent [79][69]. Innate immunity plays an important role in the aging process. The cytosolic cyclic GMP–AMP synthase (cGAS)-STING pathway is an important signaling pathway in cells whereby cytoplasmic sensory DNA activates immunity (Figure 1) [80][70]. During aging, cytoplasmic chromatin fragments (CCFs) leaked from the nucleus, and along with micronuclei or DNA that has escaped from the mitochondria, activate the cGAS-STING pathway, and thus facilitate SASP [81][71]. SASP promotes the senescence of adjacent or circulatory cells via paracrine signaling [82][72]. Recent studies have found that yes-associated protein 1 (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ)-mediated control of cGAS-STING signaling is an important molecular mechanism in the regulation of aging in stromal cells and contractile cells. YAP/TAZ is also important for maintaining nuclear envelope stability via the modulation of Lamin B1 expression [83][73].2.6. Circadian Rhythm
The production and maintenance of circadian rhythm is the result of positive and negative feedback loops regulated by a series of genes associated with the biological clock, including BMAL, CLOCK, PER, CRY, REV-ERB-α, ROR-β, etc. [84][74] (Figure 1). The circadian rhythm/clock genes are closely related to aging and two-way adjustment, with aging leading to the transcriptomic reprogramming of circadian genes. For example, the absence of the core clock transcription factor Bmal1 leads to multiple aging-like pathologies in mice [85][75]. Disturbances in the circadian rhythm accompany the occurrence of aging, and can contribute to the onset and progression of aging-related neurodegenerative diseases [86][76]. Notably, Salvador Aznar Benitah group and Sassone-Corsi group by comparing mice of different ages, revealed that a low-calorie diet can improve the circadian rhythm of somatic and stem cells, inhibiting the aging process [87,88][77][78].References
- Olovnikov, A.M. Telomeres, telomerase, and aging: Origin of the theory. Exp. Gerontol. 1996, 31, 443–448.
- Gavrilov, L.A.; Gavrilova, N.S. Evolutionary Theories of Aging and Longevity. Sci. World J. 2002, 2, 339–356.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735.
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621.
- Martínez-Zamudio, R.I.; Robinson, L.; Roux, P.-F.; Bischof, O. SnapShot: Cellular Senescence Pathways. Cell 2017, 170, 816.
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453.
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 2021, 1, 870–879.
- Singh, P.P.; Demmitt, B.A.; Nath, R.D.; Brunet, A. The Genetics of Aging: A Vertebrate Perspective. Cell 2019, 177, 200–220.
- Cagan, A.; Baez-Ortega, A.; Brzozowska, N.; Abascal, F.; Coorens, T.H.H.; Sanders, M.A.; Lawson, A.R.J.; Harvey, L.M.R.; Bhosle, S.; Jones, D.; et al. Somatic mutation rates scale with lifespan across mammals. Nature 2022, 604, 517–524.
- Gomes, N.M.V.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011, 10, 761–768.
- Muñoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 2019, 10, 4723.
- Sleiman, M.B.; Roy, S.; Gao, A.W.; Sadler, M.C.; von Alvensleben, G.V.G.; Li, H.; Sen, S.; Harrison, D.E.; Nelson, J.F.; Strong, R.; et al. Sex- and age-dependent genetics of longevity in a heterogeneous mouse population. Science 2022, 377, eabo3191.
- Lemaître, J.-F.; Ronget, V.; Tidière, M.; Allainé, D.; Berger, V.; Cohas, A.; Colchero, F.; Conde, D.A.; Garratt, M.; Liker, A.; et al. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. USA 2020, 117, 8546–8553.
- Trifunovic, A. Mitochondrial DNA and ageing. Biochim. Biophys. Acta 2006, 1757, 611–617.
- Ellegren, H. Sex-chromosome evolution: Recent progress and the influence of male and female heterogamety. Nat. Rev. Genet. 2011, 12, 157–166.
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221.
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res. 2017, 46, D1083–D1090.
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A.; et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020, 21, 91.
- Budovsky, A.; Craig, T.; Wang, J.; Tacutu, R.; Csordas, A.; Lourenço, J.; Fraifeld, V.E.; de Magalhães, J.P. LongevityMap: A database of human genetic variants associated with longevity. Trends Genet. 2013, 29, 559–560.
- Aging Atlas, C. Aging Atlas: A multi-omics database for aging biology. Nucleic Acids Res. 2021, 49, D825–D830.
- Liu, B.; Wang, J.; Chan, K.M.; Tjia, W.M.; Deng, W.; Guan, X.; Huang, J.-D.; Li, K.M.; Chau, P.Y.; Chen, D.J.; et al. Genomic instability in laminopathy-based premature aging. Nat. Med. 2005, 11, 780–785.
- Garagnani, P.; Marquis, J.; Delledonne, M.; Pirazzini, C.; Marasco, E.; Kwiatkowska, K.M.; Iannuzzi, V.; Bacalini, M.G.; Valsesia, A.; Carayol, J.; et al. Whole-genome sequencing analysis of semi-supercentenarians. Elife 2021, 10, e57849.
- Deelen, J.; Evans, D.S.; Arking, D.E.; Tesi, N.; Nygaard, M.; Liu, X.; Wojczynski, M.K.; Biggs, M.L.; van der Spek, A.; Atzmon, G.; et al. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nat. Commun. 2019, 10, 3669.
- Lin, J.-R.; Sin-Chan, P.; Napolioni, V.; Torres, G.G.; Mitra, J.; Zhang, Q.; Jabalameli, M.R.; Wang, Z.; Nguyen, N.; Gao, T.; et al. Rare genetic coding variants associated with human longevity and protection against age-related diseases. Nat. Aging 2021, 1, 783–794.
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590.
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258.
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310.
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30.
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464.
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371.
- Unnikrishnan, A.; Deepa, S.S.; Herd, H.R.; Richardson, A. Chapter 19—Extension of Life Span in Laboratory Mice. In Conn’s Handbook of Models for Human Aging, 2nd ed.; Ram, J.L., Conn, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 245–270.
- McCay, C.M.; Maynard, L.A.; Sperling, G.; Barnes, L.L. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories: Four figures. J. Nutr. 1939, 18, 1–13.
- Fontana, L.; Nehme, J.; Demaria, M. Caloric restriction and cellular senescence. Mech. Ageing Dev. 2018, 176, 19–23.
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063.
- Anderson, R.M.; Bitterman, K.J.; Wood, J.G.; Medvedik, O.; Sinclair, D.A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 2003, 423, 181–185.
- Panowski, S.H.; Wolff, S.; Aguilaniu, H.; Durieux, J.; Dillin, A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 2007, 447, 550–555.
- Vermeij, W.P.; Dollé, M.E.T.; Reiling, E.; Jaarsma, D.; Payan-Gomez, C.; Bombardieri, C.R.; Wu, H.; Roks, A.J.M.; Botter, S.M.; Van Der Eerden, B.C.; et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 2016, 537, 427–431.
- Xie, K.; Neff, F.; Markert, A.; Rozman, J.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Brommage, R.; Garrett, L.; Henzel, K.S.; et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 2017, 8, 155.
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321.
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204.
- Parsons, P.A. The limit to human longevity: An approach through a stress theory of ageing. Mech. Ageing Dev. 1996, 87, 211–218.
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517.
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009, 458, 725–731.
- Richardson, N.E.; Konon, E.N.; Schuster, H.S.; Mitchell, A.T.; Boyle, C.; Rodgers, A.C.; Finke, M.; Haider, L.R.; Yu, D.; Flores, V.; et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 2021, 1, 73–86.
- Kennedy, B.K.; Austriaco, N.R., Jr.; Zhang, J.; Guarente, L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 1995, 80, 485–496.
- Wang, T.; Wang, Y.; Liu, L.; Jiang, Z.; Li, X.; Tong, R.; He, J.; Shi, J. Research progress on sirtuins family members and cell senescence. Eur. J. Med. Chem. 2020, 193, 112207.
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie Restriction Promotes Mammalian Cell Survival by Inducing the SIRT1 Deacetylase. Science 2004, 305, 390–392.
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2019, 176, 944–945.
- Osmanagic-Myers, S.; Kiss, A.; Manakanatas, C.; Hamza, O.; Sedlmayer, F.; Szabo, P.L.; Fischer, I.; Fichtinger, P.; Podesser, B.K.; Eriksson, M.; et al. Endothelial progerin expression causes cardiovascular pathology through an impaired mechanoresponse. J. Clin. Investig. 2019, 129, 531–545.
- Sun, S.; Qin, W.; Tang, X.; Meng, Y.; Hu, W.; Zhang, S.; Qian, M.; Liu, Z.; Cao, X.; Pang, Q.; et al. Vascular endothelium–targeted Sirt7 gene therapy rejuvenates blood vessels and extends life span in a Hutchinson-Gilford progeria model. Sci. Adv. 2020, 6, eaay5556.
- Wu, X.; Liu, H.; Brooks, A.; Xu, S.; Luo, J.; Steiner, R.; Mickelsen, D.M.; Moravec, C.S.; Jeffrey, A.D.; Small, E.M.; et al. SIRT6 Mitigates Heart Failure With Preserved Ejection Fraction in Diabetes. Circ. Res. 2022, 131, 926–943.
- Liu, B.; Ghosh, S.; Yang, X.; Zheng, H.; Liu, X.; Wang, Z.; Jin, G.; Zheng, B.; Kennedy, B.K.; Suh, Y.; et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012, 16, 738–750.
- Ghosh, S.; Liu, B.; Wang, Y.; Hao, Q.; Zhou, Z. Lamin A Is an Endogenous SIRT6 Activator and Promotes SIRT6-Mediated DNA Repair. Cell Rep. 2015, 13, 1396–1406.
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Chahar, S.; Kane-Goldsmith, N.; Newkirk, S.J.; Lee, S.; Xing, J.; Verzi, M.P.; An, W.; et al. SIRT7 mediates L1 elements transcriptional repression and their association with the nuclear lamina. Nucleic Acids Res. 2019, 47, 7870–7885.
- Bi, S.; Liu, Z.; Wu, Z.; Wang, Z.; Liu, X.; Wang, S.; Ren, J.; Yao, Y.; Zhang, W.; Song, M.; et al. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell 2020, 11, 483–504.
- Kulik, G.; Weber, M.J. Akt-Dependent and -Independent Survival Signaling Pathways Utilized by Insulin-Like Growth Factor I. Mol. Cell Biol. 1998, 18, 6711–6718.
- Jiang, B.; Wu, X.; Meng, F.; Si, L.; Cao, S.; Dong, Y.; Sun, H.; Lv, M.; Xu, H.; Bai, N.; et al. Progerin modulates the IGF-1R/Akt signaling involved in aging. Sci. Adv. 2022, 8, eabo0322.
- Ibrahim, M.X.; Sayin, V.I.; Akula, M.K.; Liu, M.; Fong, L.G.; Young, S.G.; Bergo, M.O. Targeting Isoprenylcysteine Methylation Ameliorates Disease in a Mouse Model of Progeria. Science 2013, 340, 1330–1333.
- Cabral, W.A.; Tavarez, U.L.; Beeram, I.; Yeritsyan, D.; Boku, Y.D.; Eckhaus, M.A.; Nazarian, A.; Erdos, M.R.; Collins, F.S. Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome. Aging Cell 2021, 20, e13457.
- Dou, Z.; Xu, C.; Donahue, G.; Shimi, T.; Pan, J.-A.; Zhu, J.; Ivanov, A.; Capell, B.C.; Drake, A.M.; Shah, P.P.; et al. Autophagy mediates degradation of nuclear lamina. Nature 2015, 527, 105–109.
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nature 2020, 22, 1170–1179.
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254.
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Health 2013, 2, 8.
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521.
- Kim, D.H.; Bang, E.; Jung, H.J.; Noh, S.G.; Yu, B.P.; Choi, Y.J.; Chung, H.Y. Anti-Aging Effects of Calorie Restriction (CR) and CR Mimetics Based on the Senoinflammation Concept. Nutrients 2020, 12, 422.
- Wang, S.; Ge, W.; Harns, C.; Meng, X.; Zhang, Y.; Ren, J. Ablation of toll-like receptor 4 attenuates aging-induced myocardial remodeling and contractile dysfunction through NCoRI-HDAC1-mediated regulation of autophagy. J. Mol. Cell Cardiol. 2018, 119, 40–50.
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310.
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384.
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z.; et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017, 550, 402–406.
- Glück, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.-W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nature 2017, 19, 1061–1070.
- Sladitschek-Martens, H.L.; Guarnieri, A.; Brumana, G.; Zanconato, F.; Battilana, G.; Xiccato, R.L.; Panciera, T.; Forcato, M.; Bicciato, S.; Guzzardo, V.; et al. AP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature 2022, 607, 790–798.
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84.
- Janich, P.; Meng, Q.-J.; Benitah, S.A. Circadian control of tissue homeostasis and adult stem cells. Curr. Opin. Cell Biol. 2014, 31, 8–15.
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008.
- Sato, S.; Solanas, G.; Peixoto, F.O.; Bee, L.; Symeonidi, A.; Schmidt, M.S.; Brenner, C.; Masri, S.; Benitah, S.A.; Sassone-Corsi, P. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 2017, 170, 664–677.e11.
- Solanas, G.; Peixoto, F.O.; Perdiguero, E.; Jardí, M.; Ruiz-Bonilla, V.; Datta, D.; Symeonidi, A.; Castellanos, A.; Welz, P.-S.; Caballero, J.M.; et al. Aged Stem Cells Reprogram Their Daily Rhythmic Functions to Adapt to Stress. Cell 2017, 170, 678–692.e20.