Cyanobacteria, algal blooms and cyanotoxins have become common environmental enigmas in marine, freshwater and estuarine ecosystems. IThere is also compelling scientific consensus that cyanotoxins can bioaccumulate in edible aquatic organisms and affects water quality, which impacts ecosystem integrity, public health and aggravates water insecurity. Herein, this entry highlights the current knowledge on the taxonomy, bloom dynamics, toxic effects, human and ecological health implications of cyanobacteria, harmful algal blooms and cyanotoxins in the East African lakes, mainlyCommunity (EAC) lakes. Available literature shows that the major toxigenic microalgae in East African lakes belong to the Microcystis, Arthrospira, Dolichospermum, Planktolyngbya and Anabaenopsis genera. There haspeci been increased incidences of cyanobacteria have been responsible for the production of analgal blooms in eutrophic EAC lakes but there are few reports on the same in oligotrophic lakes, which indicates a gap in the current understanding of algal blooms in the region. Anatoxin-a, homoanatoxin-a, microcystins (MCs), cylindrospermopsin and nodularin have been quantified in water from below method detection limits to 81 µg L-1, with peak concentrations characteristically reported for the wet season. In edible Mifish tissues, MCs have been quantified from 2.4 to 1,479.24 μg kg-1, which can pose human health rocystinisks to a daily consumer. No episodes of human poisoning due to cyanotoxins has been reported in the EAC but MCs and anatoxin-a (up to 51.4 μg g-1) have been implicated as the proximal cause of indiscriminate fish deaths and epornitic mortality of lesser flamingos. With the unequivocal increase in climate change and variability, algal blooms and cyanotoxins will increase in frequency and severity, and this will necessitate swift action towards the mitigation of nutrient-rich pollutants loading into lakes and other water resources in the region. As some cyanobacteria encountered in EAC lakes produce other cyanotoxins (notably β-N-methylamino-L-alanine and saxitoxins), studies targeting such cyanobacterial metabolites should be initiated. Research examining the risk of hepatocellular cancer steming from ingestion of cyanobacteria and mycotoxin-contaminated water and foods, and hepatitis virus should be launched. Another potential relationship with microplastics should be assessed because they are known to accumulate toxins and amplify their toxicity
- Arthrospira fusiformis
- cylindrospermopsin
- hepatotoxicity
- lesser flamingos
- Lake Victoria
- crater lakes
1. Introduction
2. Overview of cyanobacteria, harmful algal blooms and cyanotoxins in East African lacustrine ecosystems
The East African Community (EAC), is a regional cooperation constituted by seven sovereign states as of April 2022 namely: Burundi, South Sudan, Rwanda, Democratic Republic of Congo, Tanzania, Uganda and Kenya. The region is blessed with water resources, but the water demands are largely met by eutrophic water bodies [8][9][10]. In thise regionEAC, MCs and anatoxin-a (ATX) are the main cyanotoxins that garnered early have been extensively recieved toxicological interest (Figure 1). For example, iIn the DRC, the oligotrophic Lake Tanganyika haswas reported to have CYB (Anabaenopsis species, Dolichospermum flosaquae, and Limnococcus limneticus) [11][12]. Another lake in DRC (Lake Kivu) is a deep oligotrophic and meromictic water resource with large volumes of exploitable methane in DRC (Lake Kivu) also has been cited to harbour . Nevertheless, CYB (Synechococcus species and Planktolyngbya limnetica) have been indicated to be prevalent in this lake, followed by pennate diatoms (Nitzschia bacata and Fragilaria danica) [13][14].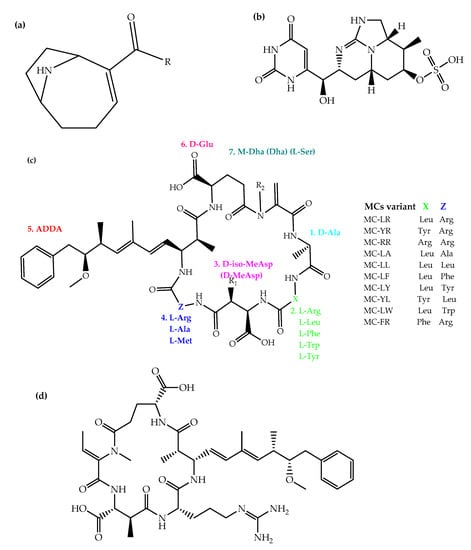
2.3. Tanzania
Pr study in Lake Naivasha confirmed the dominance of Microcystis viouspecies in a 2010 CYAHAB in the lake [20]. From a recereational perspective, a 2023 study [21] substantudiated that cyanobacterial (Microcystis as cond Dolichospermum species) cell counts in water from Winam Gulf of L. Victoria exceeded WHO standards for recreational risk in 84% of water samples. The same samples had MCs (0.02 to 23.31 ), whcih surpasses the provisional WHO drinking water guideline for MCs. In the United Republic of Tanzania, studies done on laed about lakes: Big Momela, Embagai and Manyara recorded indicated that CYB (>50%) namley:, mostly Anabaenopsis elenkenii, A. fusiformis (Lake Big Momela), A. fusiformis, Oscillatoria, Hantzschia (Lake Embagai), Oscillatoria jenensis and Pseudoanabaena terebriformis (Lake Manyara), were dominant in the phytoplankton [2220][2321]. Similarly, CYB (specifically A. fusiformis ) have equally been prevalent in Momela Lakes and Lake Natron with MCs detected in water at concentrations of 0.1–4.5 μg mL−1 of scum in the latter [2422]. In the lentic wate Fors of L. Victoria, the occurrence of CYB (upto 82%) reportwas quantified in samples from seeveral parts of the southern portion. A later investigation by art. Miles et al. [2523] ifoundicated the presence of p putative MCs analogues in extracts of a bloom sampled from Tanznaia's cyanobacterial bloom from Mwanza Gulf, but no cyanotoxin but did not quantifications were doney them. On 27 islands of Ukerewe district, MCs (0.0028 to 0.0102 μg L−1) were reported [2624]. Other studies in L. Victoria (several bays, open water and Gulfs) have found MCs (up to 13 µg MC-LR eq L−1). An incidence of multiple cyanotoxins: CYN (0.004 to 0.01 μg L−1), NODs (0.010 μg L−1) and MCs (0.0028 to 0.0118 μg L−1) in water from L. Victoria has been communicated [2624]. The report emphasized that multiple and repeated exposure to phycotoxins could amplify their toxicity and/or adverse effects.2.4. Uganda
From the available literature, Uganda has the highest number of reports about CYB in thseve regionral lakes. Of these, Western Uganda crater lakes (Kyaninga, Saaka, Nyabikere, Nyinambuga, Munyayange, Kikorongo, Maseche, Murumuli, Bunyampaka, Katwe, Bagusa, Nyamunuka, Mwamba, Katanda, Karolero, Kerere, Kacuba, Mwengenyi, Kyerbwato, Katanda, Kanyamukali, Nkugute, Kyanga, Mirambi, Nyanswiga, Kitere, Chibwera, Lugembe, Nyanswiga, Kamweru, Nyahirya, Nyabikere, Kyasanduka, Kifuruka, Wandakara, Nyamusingire, Nyungu and Katinda) were found to contain CYB (35% to 100%), primarily of the genera Planktolyngbya, Microcystis, Anabaena and Cylindrospermopsis. No determination of cyanotoxins was performed [2725][2826]. Other interesting studies are available on the Albertine lakes (Edward and George) [2927]. Species from Raphidiopsis and Anabaenopsis genera are the primary community in Lake Edward, though Aphanocapsa, Merismopedia, Microcystis, Aphanothece and Anathece genera are also present. According to several authors, shallow Ugandan lakes near Mount Rwenzori (Lake George, Lake Edward and Lake Mburo) are eutrophic, with Microcystis species being the most abundant CYB [27][28][29][30][31][32][33]. Lake Mburo was earlier reported to have more than 90% of its phytoplanktonic community as CYB [3432][3533]. In the Ugandan part of L. Victoria, Microcystis, Dolichospermum and Cylindrospermopsis species are the prevalent CYB (>80%) (Table S1). Cyanotoxin analyses have reported concentrations of NT to 93 µg L−1 of MCs in water from Murchison Bay, Napoleon gulf and open lake water. Worth citing are pioneering studies in Murchison Bay where MCs were quantified in Oreochromis niloticus (Nile tilapia fish), unveiling that the concentrations in biota and aqueous phase were correlated. The study highlighted that there has been an increase in MCs-producing CYB in the lake which are plausibly ingested by fish, agreeing with previous research findings [3331][3634][3735]. The maximum concentration of total MCs reported for guts, liver and muscles of phytoplanktivorous Oreochromis niloticus (Nile tilapia) and Lates niloticus (Nile perch) from Murchison Bay of L. Victoria (1.86 to 1479.24 μg kg−1) is slightly higher than those from other Ugandan lakes such as Lake Mburo (73.10 to 1312 μg kg−1) [3634].2.5. Rwanda
The remaining report on cyantoxins in the EAC is a single nly report on CYB fromin a Rwandese Lake (Lake Muhazi). showed that it contains mainly Microcystis aeruginosa , folloccurwed along with thby the dinoflagellate Cerutium hirundinellu [3836]. and this was confirmThese are ingested by a follow up study which Nile tilapia present in the lake [37], subggestantitated that the CYB were being ingested Nile tilapiaing the need to establish the concentrations of cyanotoxins in water and fish from the same lake [39]is lake. Overall, volcanic and tectonic lakes in the East African Great Rift Valley possess distinguished extents of hydrological connections. Volcanicity in the region resulted in endorheic basins whose bedrock, groundwater connection and climate have favored schizohaline water formation [4038]. These, in turn, have contributed to the dominance of CYB, and occurrence of CYBHAB and cyanotoxins. The present literature reveals that toxigenic microalgae recorded from EAC lakes are Dolichospermum, Microcystis, Arthrospira, Planktolyngbya and Anabaenopsis species. The prevalence of CYBHAB and cyanotoxins in EAC lakes is of concern due to potential bioaccumulation and trophic transfer in zooplanktivorous and carnivorous fish species [3331][3735]. Moreover, the observed levels of MCs in whole fish, gut, liver and muscles (2.4 to 1479.24 μg kg−1) could pose human health risks to a daily consumer, as the WHO daily intake limit of MCs in fish is 0.04 µg kg−1 [3634].3. Toxicity, Human and Ecological Health Implications of Cyanotoxins in EAC Lakes
3.1. MCs
MCs are hepatotoxins, majorly produced as secondary metabolites of planktonic cyanobacterial species from genera such as Microcystis, Cylindrospermopsis, Anabaena, Oscillatoria (Planktothrix), Anabaenopsis, Nostoc, Arthrospira, Hapalosiphon, Limnothrix, Lyngbya, Phormidium, Rivularia, Synechocystis and Synechococcus [4139]. Acute effects such as nausea, diarrhea, dermal, eye and throat irritations have been associated with their ingestion. Chronic exposure to MCs culminates in hepatic necrosis, retarded growth, reduced reproduction potential and, ultimately, death in fish and humans. The neurotoxicity of MCs is also known, but this applies specifically to invertebrates without livers [4240]. In addition, exposure to MCs is associated with colorectal and primary liver cancer, with MC-LR receiving classification as a possible human carcinogen (group 2B) [4341]. For humans, exposure to MCs occurs principally through the ingestion of contaminated aquatic organisms (e.g., fish) or water, as well as through the recreational use of water. Upon ingestion and absorption into the liver by organic anion transport proteins, MCs inhibit protein phosphatases, thereby selectively distorting cytoskeleton formation, degrading hepatic ultrastructure in eukaryotic cells, resulting in hepatic failure, intrahepatic hemorrhage and shock [4442][4543]. In EAC, MCs and ATX were implicated in the death of Phoeniconaias minor Geoffroy Saint-Hilaire 1798 (lesser flamingos) [4644]. The pink birds feed on A. fusiformis [4745], which confers upon them the pink plumage following the accumulation of ingested cyanobacterial pigments [4846]. While this phenomenon is not new (e.g., in the Greater flamingos and Western Tanager [4947][5048]), it should be anticipated that other nutrition-based compounds may become bioaccumulated in lesser flamingos, e.g., potentially toxic metals. Event-driven reports of lesser flamingo die-offs are available for soda lakes such as Bogoria and Nakuru of Kenya [5149][5250], Momela, Natron, Rishateni, Manyara and Empakai Crater of Tanzania [8][2321][5351][5452]. While it is still debated that MCs may be a potential initiator of avian botulism, other probable causes of the unnatural mass death of wild birds include avian tuberculosis [5553], cholera, botulism, heavy metals [5654][5755], pesticide residues, or combinations of these [49][56][5157][58][59][60][61]. Indeed, mycobacteriosis was reported in lesser flamingos from Lake Nakuru, Kenya [6260]. Nevertheless, anatoxins and MCs (at concentrations higher than reported in EAC flamingos) have been associated with avian mortalities [5856][61][62][63][64][65][66].3.2. Anatoxin-a
ATX is toxicologically known as Very Fast Death Factor for its fast lethal effect in animals, which could be related to its high rate of absorption into the gastrointestinal tract [6765]. ATX is a secondary bicyclic amine alkaloid with peracute neurotoxic effects. Its discovery and identification in the 1960s and 1972 from CYB (Anabaena flos-aquae) followed the mortality of cattle herds that ingested contaminated water from Saskatchewan Lake in Ontario [6765]. It is known to be biosynthesized by CYB from Arthrospira, Anabaena, Microcystis, Planktothrix, Oscillatoria, Aphanizomenon and Cylindrospermum genus [6765]. Exposure to ATX (through ingestion of contaminated water or dried algal crusts, accidental swallowing/inhalation) has been associated with burning, tingling, respiratory paralysis and dysrhythmias, which are fatal. ATX antagonizes the activity of neuronal α4β2 and α4 nicotinic acetylcholine receptors (nAchRs) of the central nervous system and (α1)2βγδ muscle-type nAchRs of the neuromuscular junction [6866]. With an affinity >20 times that of acetylcholine, ATX has the same effect as the former when it binds with nAchRs, i.e., it induces a conformational effect on the receptor, opening the channel pore to permit the passage of ions (Ca2+ and Na+) into the neuron. This culminates into cell depolarization, the generation of action potentials and thus muscle contraction. During ATX-mediated toxicity, the acetylcholine neurotransmitter does not dissociate from the nAchRs, resulting into irreversible inhibition and blockage of neuromuscular transmission [6967].3.3. Homoanatoxin-a
HATX being structurally a higher homologue of ATX has the same toxic effects as ATX. In addition to its nicotinic agonistic effects, HATX also upregulates acetylcholine release from cholinergic nerves [7068]. This may explain why the potency of HATX is greater than that of ATX. Mortalities from CYBHAB with HATX are rare, but a report of dog neurotoxicosis from New Zealand (where the animals ingested CYB from Hutt River, lower North Island with 4400 µg kg−1 wet weight of HATX) has been published [7169].3.4. Cylindrospermopsin
CYN is a hydrophilic potentially hepatotoxic and immunotoxic cyclic guanidinium alkaloid, with characteristic tricyclic hydroxymethyl uracil [4240]. It has some analogues such as deoxy-CYN (lacking an oxygen atom), demethoxy-CYN and 7-epiCYN (difference in the orientation of hydroxyl group) isolated in CYB Cylindrospermopsis raciborskii. The discovery of CYN toxicity happened when more than 100 children from Palm Island in Queensland, Australia suffered from unprecedented gastroenteritis and hepatomegaly. The ordeal was finally found to be due to the ingestion of CYN in contaminated water with CYBHAB of C. raciborskii [7270]. However, CYN is also produced by other CYB, including Aphanizomenon flos-aquae, Anabaena species (bergii, and lapponica), Aphanizomenon ovalisporum, Lyngbya wollei, Raphidiopsis curvata Oscillatoria (Planktothrix) species and Umezakia natans [4139]. With guideline values of 0.5 to 3 μg L−1 in drinking water across continents, CYN is the second-most-studied cyanotoxin known to target the liver, kidneys, heart, spleen, ovary, eye, lung, T lymphocytes, neutrophils and vascular endothelium [7371]. CYN elicit toxicity through inhibition of protein synthesis, which can also occur at subtoxic concentrations [7472]. Other toxicologists stated that CYN (with its inherent reactive guanidine) could be largely toxic through the induction of DNA wreckage and disruption of the kinetochore spindle. This could possibly result in chromosome loss, aneugenic and clastogenic effects [7573]. Chichova et al. [7371] found that CYN elicited moderate toxicity in human intestinal epithelial cells with suppression of cellular regeneration of the epithelial layer. CYN shows hepatotoxic, nephrotoxic, and cytotoxic effects, suggesting potential carcinogenicity. The neurotoxic potential of CYN has also been cited, though this could be a direct consequence of its cytotoxicity. To this end, the full underlying mechanisms of CYN toxicity needs to be elucidated [4240]. In the EAC, there are no toxicity reports on CYN, which may be due to the absence of robust data on this cyanotoxin. There are, however, episodes of human and animal CYN-related poisoning from other countries. The most notable human poisoning is the 1979 Solomon dam gastroenteritis and hepatomegaly incidence in children from Palm Island [7270]. The mortality of a cow and three calves after drinking water from McKinley Shire dam, Northern Queensland (Australia) was also reported. The animals had severe abdominal and thoracic haemorrhagic effusion, hyperaemic mesentery, pale and swollen liver, extremely distended gall bladder with dark yellow bile and epicardial haemorrhages [7674]. In the subsequent 21 days, another eight animals (two cows and six calves) died, and analyses implicated CYN in C. raciborskii as the cause [7674]. In Lake Aleksandrovac (Serbia), indiscriminate fish deaths due to the ingestion of CYN (range: 1.91 and 24.28 µg L−1) were reported [7775]. This report may point to the need to establish CYN levels in EAC lakes where indiscriminate fish deaths have been reported, as CYN may be a contributing factor in addition to MCs.3.5. Nodularins
Nodularins, a class of hepatotoxic non-ribosomal cyclic pentapeptides, possess toxicity mechanisms similar to those of MCs [7876]. They are structurally analogous to MCs, but differentiable from MCs in their amino acid components. To date, ten naturally occurring variants (isoforms) of NODs have been discovered, but nodularin-R (with Z amino acid = arginine) is the most common, most commercially available and most studied variant. The toxicity of NODs mainly targets the liver, but they also accumulate in the intestines, blood and kidneys [7977]. Upon ingestion, NODs diffuse from the proximal and distal ileum into the liver [8078], where they inhibit active sites of serine/threonine protein phosphatases (PP) namely: 1 (PP-1), 2A (PP-2A) and 3 (PP-3). A non-covalent interaction occurs at first with the side chain (ADDA part) and a free D-glutamyl carboxyl group in the cyclic structure of the PP, followed by the inhibition of the phosphatase activities. NODs–phosphatase complexes (NODs-PP-1 and NODs-PP-2A) are formed with exceptionally stable bonds. Thus, the key difference between NODs and MCs in their toxicity via protein phosphatases inhibition is that the former binds non-covalently to phosphatases, while the latter forms a covalent bond [7977]. Also, NODs also elicit toxicity through formation of superoxide and hydroxyl radicals (reactive oxygen species) according to a yet incompletely elucidated pathway [7977]. Their tumor-promoting activity is, on the other hand, mediated through the induced gene expression of TNF-alpha and proto-oncogenes, the exact mechanism of which is yet to be unraveled. In addition, the deactivation of the resultant tumor suppressor gene products (retinoblastoma and p53) progresses via phosphorylation, and this inevitably promotes tumorigenesis [8179]. Overall, the cascade of reactions following NOD ingestion causes cellular disorganizations and damages, apoptosis, necrosis, loss of cell integrity, DNA fragmentation and strand breaks, intrahepatic bleeding and rapid blistering of hepatocytes which results in blood pooling and doubling of the liver weight [8280]. Thus, mortalities associated with NOD poisoning is mediated through hemorrhagic shocks, which occurs in a few hours when ingested at high concentrations [8381]. There are no toxicity events involving NODs in the EAC. Nevertheless, animal (cattle, dog, sheep, horse, pig and guinea pig) NOD-poisoning-related mortalities have been reported in other parts of the world. For example, hepatotoxicosis of a South African dog following the ingestion of NODs (0.00000347 µg kg−1 DW) was reported [8482]. This emphasizes that more studies on this cyanotoxin is warranted in EAC aquatic ecosystemsI
4. Conclusions
Cyan conclusion, CYB, bacteria, algal blooms and cyanotoxins have increased in East African lacustrine ecosystems. Dolichospermum, Microcystis, Arthrospira, Planktolyngbya and Anabaenopsis species are the major groups of CYB prevalent in EAC lakes producing ATX, HATX, MCs, CYN and NODs. Shallow EAC lakes exhibit less seasonality in their CYB composition, with Microcystis being the CYB producing MCs under shallow and eutrophic lacustrine conditions. The only direct ecological effects of cyanotoxins in EAC lakes is indiscriminate fish deaths and mass die-offs of lesser flamingos. With the unequivocal increase in climate change and variability, it is inferred that CYBHAB and cyanotoxins will increase in frequency and severity. This calls for urgent action to mitigate nutrient-rich pollutants loading into water resources and the expansion of CYBHAB from eutrophic lakes to the surrounding marine environments. The (eco)toxicological relevance of co-production of phycotoxins should be assessed in the EAC because such exposure may amplify the toxicological outcomes in aquatic biota and humans. As some CYB encountered in EAC lakes produce other cyanotoxins (such as β-N-methylamino-L-alanine and saxitoxins), studies targeting these cyanobacterial metabolites should be initiated. While there are no reports of cyanotoxin poisoning of humans in the EAC, future studies should examine the risk of hepatocellular cancer, the ingestion of CYB and mycotoxin-contaminated water and foods, and hepatitis virus, which were earlier linked to increased primary liver cancer cases in Asia. Another potential relationship with microplastics should be assessed because they are known to accumulate toxins and amplify their toxicity.
References
- Band-Schmidt, C.; Durán-Riveroll, L.; Bustillos-Guzmán, J.; Leyva-Valencia, I.; López-Cortés, D.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.; Ramírez-Rodríguez, D. Paralytic Toxin Producing Dinoflagellates in Latin America: Ecology and Physiology. Front. Mar. Sci. 2019, 6, 42.
- Klijnstra, M.D.; Faassen, E.J.; Gerssen, A. A Generic LC-HRMS Screening Method for Marine and Freshwater Phycotoxins in Fish, Shellfish, Water, and Supplements. Toxins 2021, 13, 823.
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae 2016, 60, 11–26.
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; Yokota, K.; Zhan, Q. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859.
- Kat, M. Special meeting on causes, dynamics and effects of exceptional marine blooms and related events. Int. Counc. Explor. Sea C 1984, 3.
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017.
- Gerssen, A.; Gago-Martinez, A. Emerging marine biotoxins. Toxins 2019, 11, 314.
- Lihepanyama, D.L.; Ndakidemi, P.A.; Treydte, A.C. Spatio–TemporalWater Quality Determines Algal Bloom Occurrence and Possibly Lesser Flamingo (Phoeniconaias minor) Presence in Momella Lakes, Tanzania. Water 2022, 14, 3532.
- Olokotum, M.; Mitroi, V.; Troussellier, M.; Semyaloa, R.; Bernarde, C.; Montuelle, B.; Okello, W.; Quiblier, C.; Humbert, J.-F. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 2020, 96, 101829.
- Saulnier-Talbot, É.; Gregory-Eaves, I.; Simpson, K.; Efitre, J.; Nowlan, T.; Taranu, Z.; Chapman, L. Small Changes in Climate Can Profoundly Alter the Dynamics and Ecosystem Services of Tropical Crater Lakes. PLoS ONE 2014, 9, e86561.
- Cocquyt, C.; Plisnier, P.-D.; Mulimbwa, N.; Nshombo, M. Unusual massive phytoplankton bloom in the oligotrophic Lake Tanganyika. Plant Ecol. Evol. 2021, 154, 351–361.
- Ehrenfels, B.; Bartosiewicz, M.; Mbonde, A.; Baumann, K.; Dinkel, C.; Junker, J.; Kamulali, T.; Kimirei, I.; Niederdorfer, R.; Odermatt, D.; et al. Diazotrophic Cyanobacteria are Associated With a Low Nitrate Resupply to Surface Waters in Lake Tanganyika. Front. Environ. Sci 2021, 9, 716765.
- Sarmento, H.; Darchambeau, F.; Descy, J. Phytoplankton of Lake Kivu; Descy, J.P., Darchambeau, F., Schmid, M., Eds.; Lake Kivu. Aquatic Ecology Series; Springer: Dordrecht, The Netherlands, 2012; Volume 5, pp. 67–83.
- Rugema, E.; Darchambeau, F.; Sarmento, H.; Stoyneva-Gärtner, M.; Leitao, M.; Thiery, W.; Latli, A.; Descy, J.P. Long-term change of phytoplankton in Lake Kivu: The rise of the greens. Freshw. Biol. 2019, 64, 1940–1955.
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. J. Plankton Res. 2004, 26, 925–935.
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in the crater lakes Sonachi and Simbi, Kenya. Harmful Algae 2005, 4, 139–150.
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (L. Victoria, Kenya). Hydrobiologia 2012, 691, 109–122.
- Githukia, C.; Onyango, D.; Lusweti, D.; Ramkat, R.; Kowenje, C.; Miruka, J.; Lung’ayia, H.; Orina, P. An Analysis of Knowledge, Attitudes and Practices of Communities in Lake Victoria, Kenya on Microcystin Toxicity. Open J. Ecol. 2022, 12, 198–210.
- Roegner, A.; Sitoki, L.; Weirich, C.; Corman, J.; Owage, D.; Umami, M.; Odada, E.; Miruka, J.; Ogari, Z.; Smith, W.; et al. Harmful Algal Blooms Threaten the Health of Peri-Urban Fisher Communities: A Case Study in Kisumu Bay, Lake Victoria, Kenya. Expo. Health 2020, 12, 835–848.
- Raffoul, Melissa H., "Assessing the potential health risk of cyanobacteria harmful algal blooms andcyanotoxins in Lake Naivasha, Kenya" (2012). Electronic Thesis and Dissertation Repository. 828.https://ir.lib.uwo.ca/etd/828Hamisi, M.; Lugomela, C.; Lyimo, T.; Bergman, B.; Díez, B. Plankton composition, biomass, phylogeny and toxin genes in Lake Big Momela, Tanzania. Afr. J. Aquat. Sci. 2017, 42, 109–121.
- Roegner, A. F., J. R. Corman, L. M. Sitoki, Z. A. Kwena, Z. Ogari, J. B. Miruka, A. Xiong, C. Weirich, C. M. Aura, and T. R. Miller Impacts of algal blooms and microcystins in fish on small-scale fishers in Winam Gulf, Lake Victoria: implications for health and livelihood. Ecology and Society 2023, 28, 49, https://doi.org/10.5751/ES-13860-280149.Lugomela, C.; Pratap, H.B.; Mgaya, Y.D. Cyanobacteria Blooms: A Possible Cause of Mass Mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 2006, 5, 534–541.
- Hamisi, M.; Lugomela, C.; Lyimo, T.; Bergman, B.; Díez, B. Plankton composition, biomass, phylogeny and toxin genes in Lake Big Momela, Tanzania. Afr. J. Aquat. Sci. 2017, 42, 109–121. Nonga, H.E.; Mdegela, R.H.; Sandvik, M.; Lie, E.; Miles, C.O.; Skaare, J.U. Cyanobacteria and cyanobacterial toxins in the alkaline-saline Lakes Natron and Momela, Tanzania. Tanzan. Vet. Assoc. Proc. 2017, 32, 108–116.
- Lugomela, C.; Pratap, H.B.; Mgaya, Y.D. Cyanobacteria Blooms: A Possible Cause of Mass Mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 2006, 5, 534–541. Miles, C.O.; Sandvik, M.; Nonga, H.E.; Rundberget, T.; Wilkins, A.L.; Rise, F.; Ballot, A. Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC-MS with thiol derivatization. Toxicon 2013, 70, 21–31.
- Nonga, H.E.; Mdegela, R.H.; Sandvik, M.; Lie, E.; Miles, C.O.; Skaare, J.U. Cyanobacteria and cyanobacterial toxins in the alkaline-saline Lakes Natron and Momela, Tanzania. Tanzan. Vet. Assoc. Proc. 2017, 32, 108–116. Mchau, G.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.; Mpolya, E.; Meneely, J.; Elliott, C.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2021, 13, 185–194.
- Miles, C.O.; Sandvik, M.; Nonga, H.E.; Rundberget, T.; Wilkins, A.L.; Rise, F.; Ballot, A. Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC-MS with thiol derivatization. Toxicon 2013, 70, 21–31. Busobozi, E. Eutrophication in Ugandan Crater Lakes. A Case Study of Six Crater Lakes Located in Kabarole District Western Uganda. Master’s Thesis, University of Canterbury Christchurch, Christchurch, New Zealand, 2017.
- Mchau, G.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.; Mpolya, E.; Meneely, J.; Elliott, C.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2021, 13, 185–194. Nankabirwa, A.; De Crop, W.; Van der Meeren, T.; Cocquyt, C.; Plisnier, P.-D.; Balirwa, J.; Verschuren, D. Phytoplankton communities in the crater lakes of western Uganda, and their indicator species in relation to lake trophic status. Ecol. Indic. 2019, 107, 105563.
- Busobozi, E. Eutrophication in Ugandan Crater Lakes. A Case Study of Six Crater Lakes Located in Kabarole District Western Uganda. Master’s Thesis, University of Canterbury Christchurch, Christchurch, New Zealand, 2017. Stoyneva-Gärtner, M.P.; Morana, C.; Borges, A.V.; Okello, W.; Bouillon, S.; Deirmendjian, L.; Lambert, T.; Roland, F.; Nankabirwa, A.; Nabafu, E.; et al. Diversity and ecology of phytoplankton in Lake Edward (East Africa): Present status and long-term changes. J. Great Lakes Res. 2020, 46, 741–751.
- Nankabirwa, A.; De Crop, W.; Van der Meeren, T.; Cocquyt, C.; Plisnier, P.-D.; Balirwa, J.; Verschuren, D. Phytoplankton communities in the crater lakes of western Uganda, and their indicator species in relation to lake trophic status. Ecol. Indic. 2019, 107, 105563. Ganf, G.G. Phytoplankton Biomass and Distribution in a Shallow Eutrophic Lake (Lake George, Uganda). Oecologia 1974, 16, 9–29.
- Stoyneva-Gärtner, M.P.; Morana, C.; Borges, A.V.; Okello, W.; Bouillon, S.; Deirmendjian, L.; Lambert, T.; Roland, F.; Nankabirwa, A.; Nabafu, E.; et al. Diversity and ecology of phytoplankton in Lake Edward (East Africa): Present status and long-term changes. J. Great Lakes Res. 2020, 46, 741–751. Okello, W.; Ostermaier, V.; Portmann, C.; Gademann, K.; Kurmayer, R. Spatial isolation favours the divergence in microcystin net production by Microcystis in Ugandan freshwater lakes. Water Res. 2010, 44, 2803–2814.
- Ganf, G.G. Phytoplankton Biomass and Distribution in a Shallow Eutrophic Lake (Lake George, Uganda). Oecologia 1974, 16, 9–29. Kamanyi, J.R.; Ogwang, O.; Twongo, E. Plankton identified in stomach contents of Oreochromis niloticus (Pisces, Cichlidae) and the water system of Lakes Edward, George, and Kazinga channel—Uganda. Afr. J. Trop. Hydrobiol. Fish 1996, 7, 49–54.
- Okello, W.; Ostermaier, V.; Portmann, C.; Gademann, K.; Kurmayer, R. Spatial isolation favours the divergence in microcystin net production by Microcystis in Ugandan freshwater lakes. Water Res. 2010, 44, 2803–2814. Nyakoojo, C.; Byarujali, S.M. An ecological study of two shallow, equatorial lakes: Lake Mburo and Lake Kachera, Uganda. Afr. J. Ecol. 2010, 48, 860–864.
- Kamanyi, J.R.; Ogwang, O.; Twongo, E. Plankton identified in stomach contents of Oreochromis niloticus (Pisces, Cichlidae) and the water system of Lakes Edward, George, and Kazinga channel—Uganda. Afr. J. Trop. Hydrobiol. Fish 1996, 7, 49–54. Kayiira, D. Algal Community of Lake Mburo and Murchison Bay, Lake Victoria. Master’s Thesis, Makerere University, Kampala, Uganda, 2007.
- Nyakoojo, C.; Byarujali, S.M. An ecological study of two shallow, equatorial lakes: Lake Mburo and Lake Kachera, Uganda. Afr. J. Ecol. 2010, 48, 860–864. Byarujali, S.M. Phytoplankton production in L. Mburo—Western Uganda. In Proceedings of the First Conference on Ecology and Sustainable Natural Resource Management for Development, Mweya, Queen Elizabeth National Park, Uganda, 27 February–3 March 1995; pp. 284–290.
- Kayiira, D. Algal Community of Lake Mburo and Murchison Bay, Lake Victoria. Master’s Thesis, Makerere University, Kampala, Uganda, 2007. Nyakairu, G.; Nagawa, C.; Mbabazi, J. Assessment of cyanobacteria toxins in freshwater fish: A case study of Murchison Bay (Lake Victoria) and Lake Mburo, Uganda. Toxicon 2010, 55, 939–946.
- Byarujali, S.M. Phytoplankton production in L. Mburo—Western Uganda. In Proceedings of the First Conference on Ecology and Sustainable Natural Resource Management for Development, Mweya, Queen Elizabeth National Park, Uganda, 27 February–3 March 1995; pp. 284–290. Semyalo, R.; Rohrlack, T.; Naggawa, C.; Nyakairu, G.W. Microcystin concentrations in Nile Tilapia (Oreochromis niloticus) caught from Murchison Bay, L. Victoria and Lake Mburo: Uganda. Hydrobiologia 2009, 638, 235–244.
- Nyakairu, G.; Nagawa, C.; Mbabazi, J. Assessment of cyanobacteria toxins in freshwater fish: A case study of Murchison Bay (Lake Victoria) and Lake Mburo, Uganda. Toxicon 2010, 55, 939–946. Mukankomeje, R.; Plisnier, P.-D.; Descy, J.-P.; Massaut, L. Lake Muzahi, Rwanda: Limnological features and phytoplankton production. Hydrobiologia 1993, 257, 107–120.
- Semyalo, R.; Rohrlack, T.; Naggawa, C.; Nyakairu, G.W. Microcystin concentrations in Nile Tilapia (Oreochromis niloticus) caught from Murchison Bay, L. Victoria and Lake Mburo: Uganda. Hydrobiologia 2009, 638, 235–244. Mukankomeje, R.; Laviolette, F.; Descy, J.-P. Régime alimentaire de Tilapia, Oreochromis niloticus, du Lac Muhazi (Rwanda). Ann. De Limnol. 1994, 30, 297–312.
- Mukankomeje, R.; Plisnier, P.-D.; Descy, J.-P.; Massaut, L. Lake Muzahi, Rwanda: Limnological features and phytoplankton production. Hydrobiologia 1993, 257, 107–120. Fazi, S.; Butturini, A.; Tassi, F.; Amalfitano, S.; Venturi, S.; Vazquez, E.; Clokie, M.; Wanjala, S.W.; Pacini, N.; Harper, D.M. Biogeochemistry and biodiversity in a network of saline–alkaline lakes: Implications of ecohydrological connectivity in the Kenyan Rift Valley. Ecohydrol. Hydrobiol. 2018, 18, 96–106.
- Mukankomeje, R.; Laviolette, F.; Descy, J.-P. Régime alimentaire de Tilapia, Oreochromis niloticus, du Lac Muhazi (Rwanda). Ann. De Limnol. 1994, 30, 297–312. Rastogi, R.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol 2015, 6, 1254.
- Fazi, S.; Butturini, A.; Tassi, F.; Amalfitano, S.; Venturi, S.; Vazquez, E.; Clokie, M.; Wanjala, S.W.; Pacini, N.; Harper, D.M. Biogeochemistry and biodiversity in a network of saline–alkaline lakes: Implications of ecohydrological connectivity in the Kenyan Rift Valley. Ecohydrol. Hydrobiol. 2018, 18, 96–106. Diez-Quijada, L.; Benítez-González, M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins 2021, 13, 711.
- Rastogi, R.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol 2015, 6, 1254. International Agency for Research on Cancer (IARC). Microcystin-LR. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 2 December 2022).
- Diez-Quijada, L.; Benítez-González, M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins 2021, 13, 711. Otero, P.; Silva, M. The role of toxins: Impact on human health and aquatic environments. In the Pharmacological Potential of Cyanobacteria; Academic Press: Cambridge, MA, USA, 2022; pp. 173–199.
- International Agency for Research on Cancer (IARC). Microcystin-LR. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 2 December 2022).WHO. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; WHO/HEP/ECH/WSH/2020.6; Available online: https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf (accessed on 2 December 2022).
- Otero, P.; Silva, M. The role of toxins: Impact on human health and aquatic environments. In the Pharmacological Potential of Cyanobacteria; Academic Press: Cambridge, MA, USA, 2022; pp. 173–199. Codd, G.A.; Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K. Susceptibility of flamingos to cyanobacterial toxins via feeding. Vet. Rec. 2003, 152, 722–723.
- WHO. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; WHO/HEP/ECH/WSH/2020.6; Available online: https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf (accessed on 2 December 2022).Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 36831.
- Codd, G.A.; Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K. Susceptibility of flamingos to cyanobacterial toxins via feeding. Vet. Rec. 2003, 152, 722–723. Fox, D.L.; McBeth, J.W. Some dietary carotenoids and blood-carotenoid levels in flamingos. Comp. Biochem. Physiol. 1970, 34, 707–713.
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 36831. Fox, D.; Smith, V.E.; Wolfson, A.A. Carotenoid selectivity in blood and feathers of lesser (African), Chilean and greater (European) flamingos. Comp. Biochem. Physiol. 1967, 23, 225–232.
- Fox, D.L.; McBeth, J.W. Some dietary carotenoids and blood-carotenoid levels in flamingos. Comp. Biochem. Physiol. 1970, 34, 707–713. Lewis, D.; Metallinos-Katsaras, E.; Grivetti, L. Coturnism: Human Poisoning By European Migratory Quail. J. Cult. Geogr. 1987, 7, 51–65.
- Fox, D.; Smith, V.E.; Wolfson, A.A. Carotenoid selectivity in blood and feathers of lesser (African), Chilean and greater (European) flamingos. Comp. Biochem. Physiol. 1967, 23, 225–232. Koenig, R. The pink death: Die-offs of the lesser flamingo raise concern. Science 2006, 313, 1724–1725.
- Lewis, D.; Metallinos-Katsaras, E.; Grivetti, L. Coturnism: Human Poisoning By European Migratory Quail. J. Cult. Geogr. 1987, 7, 51–65. Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K.; Krause, E.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Krienitz, L. Cyanobacterial toxins, a further contributory cause of mass deaths of flamingos at Kenyan Rift Valley lakes. In Abstracts of the Xth International Conference on Harmful Algae; Tradewinds Conference Center: St. Pete Beach, FL, USA, 2002; p. 20.
- Koenig, R. The pink death: Die-offs of the lesser flamingo raise concern. Science 2006, 313, 1724–1725. Nonga, H.; Sandvik, M.; Miles, C.; Lie, E.; Mdegela, R.; Mwamengele, G.; Semuguruka, W.; Skaare, J. Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678, 167–178.
- Ballot, A.; Pflugmacher, S.; Wiegand, C.; Kotut, K.; Krause, E.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Krienitz, L. Cyanobacterial toxins, a further contributory cause of mass deaths of flamingos at Kenyan Rift Valley lakes. In Abstracts of the Xth International Conference on Harmful Algae; Tradewinds Conference Center: St. Pete Beach, FL, USA, 2002; p. 20. Fyumagwa, R.D.; Bugwesa, Z.; Mwita, M.; Kihwele, E.S.; Nyaki, A.; Mdegela, R.H.; Mpanduji, D.G. Cyanobacterial toxins and bacterial infections are the possible causes of mass mortality of lesser flamingos in Soda lakes in northern Tanzania. Res. Opin. Anim. Vet. Sci. 2013, 3, 1–6.
- Nonga, H.; Sandvik, M.; Miles, C.; Lie, E.; Mdegela, R.; Mwamengele, G.; Semuguruka, W.; Skaare, J. Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678, 167–178. Kock, N.D.; Kock, R.A.; Wambua, J.; Kamau, G.J.; Mohan, K. Mycobacterium avium related epizootic in free ranging Lesser Flamingos. Kenya J. Wildl. Dis. 1999, 35, 297–300.
- Fyumagwa, R.D.; Bugwesa, Z.; Mwita, M.; Kihwele, E.S.; Nyaki, A.; Mdegela, R.H.; Mpanduji, D.G. Cyanobacterial toxins and bacterial infections are the possible causes of mass mortality of lesser flamingos in Soda lakes in northern Tanzania. Res. Opin. Anim. Vet. Sci. 2013, 3, 1–6. Ndetei, R.; Muhandiki, V.S. Mortalities of Lesser Flamingos in Kenyan Rift Valley saline lakes and the implications for sustainable management of the lakes. Lakes Reserv. Res. Manag. 2005, 10, 51–58.
- Kock, N.D.; Kock, R.A.; Wambua, J.; Kamau, G.J.; Mohan, K. Mycobacterium avium related epizootic in free ranging Lesser Flamingos. Kenya J. Wildl. Dis. 1999, 35, 297–300. Nelson, Y.M.; Thampy, R.J.; Motellin, G.K.; Raini, J.A.; Disante, C.J.; Lion, L.W. Model for trace metal exposure in filter feeding flamingo at an alkaline rift valley lake, Kenya. Environ. Toxicol. Chem. 1998, 17, 2302–2309.
- Ndetei, R.; Muhandiki, V.S. Mortalities of Lesser Flamingos in Kenyan Rift Valley saline lakes and the implications for sustainable management of the lakes. Lakes Reserv. Res. Manag. 2005, 10, 51–58. Foss, A.J.; Miles, C.O.; Samdal, I.A.; Løvberg, K.E.; Wilkins, A.L.; Rise, F.; Jaabæk, J.A.H.; McGowan, P.C.; Aubel, M.T. Analysis of free and metabolized microcystins in samples following a bird mortality event. Harmful Algae 2018, 80, 117–129.
- Nelson, Y.M.; Thampy, R.J.; Motellin, G.K.; Raini, J.A.; Disante, C.J.; Lion, L.W. Model for trace metal exposure in filter feeding flamingo at an alkaline rift valley lake, Kenya. Environ. Toxicol. Chem. 1998, 17, 2302–2309. Sileo, L.; Grootenhuise, J.G.; Tuite, G.H.; Hopcraft, H.D. Microbacteriosis in the Lesser Flamingo of Lake Nakuru, Kenya. J. Wildl. Dis. 1979, 15, 387–390.
- Foss, A.J.; Miles, C.O.; Samdal, I.A.; Løvberg, K.E.; Wilkins, A.L.; Rise, F.; Jaabæk, J.A.H.; McGowan, P.C.; Aubel, M.T. Analysis of free and metabolized microcystins in samples following a bird mortality event. Harmful Algae 2018, 80, 117–129. Miller, E.; Brunner, E.; Driscoll, C.; McGowan, P. Botulism.Or Is It? Wildl. Rehabil. Bull. 2013, 31, 1–12.
- Sileo, L.; Grootenhuise, J.G.; Tuite, G.H.; Hopcraft, H.D. Microbacteriosis in the Lesser Flamingo of Lake Nakuru, Kenya. J. Wildl. Dis. 1979, 15, 387–390. WWF–LNCDP. Annual Report. World Wide Fund for Nature–Lake Nakuru Conservation and Development Project (WWF–LNCDP); WWF–LNCDP: Nakuru, Kenya, 1994.
- Miller, E.; Brunner, E.; Driscoll, C.; McGowan, P. Botulism.Or Is It? Wildl. Rehabil. Bull. 2013, 31, 1–12. IUCN. Flamingo. Bulletin of the IUCN-SSC/Wetlands International. 2015. Available online: https://www.wetlands.org/wp-content/uploads/2015/11/Flamingo-Newsletter-14-2006.pdf (accessed on 1 December 2022).
- WWF–LNCDP. Annual Report. World Wide Fund for Nature–Lake Nakuru Conservation and Development Project (WWF–LNCDP); WWF–LNCDP: Nakuru, Kenya, 1994. Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: Sudden appearance of toxic cyanobacteria. Nat. Toxins 1999, 7, 81–84.
- IUCN. Flamingo. Bulletin of the IUCN-SSC/Wetlands International. 2015. Available online: https://www.wetlands.org/wp-content/uploads/2015/11/Flamingo-Newsletter-14-2006.pdf (accessed on 1 December 2022).Henriksen, P.; Carmichael, W.W.; An, J.; Moestrup, Ø. Detection of an anatoxin-a (s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in the stomach contents of poisoned birds. Toxicon 1997, 35, 901–913.
- Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: Sudden appearance of toxic cyanobacteria. Nat. Toxins 1999, 7, 81–84. Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Envirn. Pollut. 2018, 234, 779–787.
- Henriksen, P.; Carmichael, W.W.; An, J.; Moestrup, Ø. Detection of an anatoxin-a (s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in the stomach contents of poisoned birds. Toxicon 1997, 35, 901–913. Driscoll, C.; McGowan, P.; Miller, E.; Carmichael, W. Case Report: Great blue heron (Ardea herodias) morbidity and mortality investigation in Maryland’s Chesapeake Bay. In Proceedings of the Southeast Fish and Wildlife Conference, Baltimore, MD, USA, 24 October 2002.
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Envirn. Pollut. 2018, 234, 779–787. Botana, L.; James, K.; Crowley, J.; Duphard, J.; Lehane, M.; Furey, A. Anatoxin-a and Analogues: Discovery, Distribution, and Toxicology. In Phycotoxins: Chemistry and Biochemistry; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 141–158.
- Driscoll, C.; McGowan, P.; Miller, E.; Carmichael, W. Case Report: Great blue heron (Ardea herodias) morbidity and mortality investigation in Maryland’s Chesapeake Bay. In Proceedings of the Southeast Fish and Wildlife Conference, Baltimore, MD, USA, 24 October 2002. Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828.
- Botana, L.; James, K.; Crowley, J.; Duphard, J.; Lehane, M.; Furey, A. Anatoxin-a and Analogues: Discovery, Distribution, and Toxicology. In Phycotoxins: Chemistry and Biochemistry; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 141–158. Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089.
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. Aas, P.; Eriksen, S.; Kolderup, J.; Lundy, P.; Haugen, J.E.; Skulberg, O.M.; Fonnum, F. Enhancement of acetylcholine release by homoanatoxin-a from Oscillatoria formosa. Environ. Toxicol. Pharmacol. 1996, 2, 223–232.
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301.
- Aas, P.; Eriksen, S.; Kolderup, J.; Lundy, P.; Haugen, J.E.; Skulberg, O.M.; Fonnum, F. Enhancement of acetylcholine release by homoanatoxin-a from Oscillatoria formosa. Environ. Toxicol. Pharmacol. 1996, 2, 223–232. Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. Chem. 2003, 18, 78–93.
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. Chichova, M.; Tasinov, O.; Shkodrova, M.; Mishonova, M.; Sazdova, I.; Ilieva, B.; Doncheva-Stoimenova, D.; Kiselova-Kaneva, Y.; Raikova, N.; Uzunov, B.; et al. New Data on Cylindrospermopsin Toxicity. Toxins 2021, 13, 41.
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. Chem. 2003, 18, 78–93. Froscio, S.; Humpage, A.; Wickramasinghe, W.; Shaw, G.; Falconer, I. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 2008, 51, 191–198.
- Chichova, M.; Tasinov, O.; Shkodrova, M.; Mishonova, M.; Sazdova, I.; Ilieva, B.; Doncheva-Stoimenova, D.; Kiselova-Kaneva, Y.; Raikova, N.; Uzunov, B.; et al. New Data on Cylindrospermopsin Toxicity. Toxins 2021, 13, 41. Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000, 472, 155–161.
- Froscio, S.; Humpage, A.; Wickramasinghe, W.; Shaw, G.; Falconer, I. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 2008, 51, 191–198. Thomas, A.D.; Saker, M.L.; Norton, J.H.; Olsen, R.D. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust. Vet. J 1998, 76, 592–594.
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000, 472, 155–161. Đorđević, N.; Simić, S.; Ćirić, A. First identification of the cylindrospermopsin (cyn)-producing cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju in Serbia. Fresenius Env. Bull. 2015, 24, 3736–3742.
- Thomas, A.D.; Saker, M.L.; Norton, J.H.; Olsen, R.D. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Aust. Vet. J 1998, 76, 592–594. Beasley, V.R. Harmful Algal Blooms (Phycotoxins). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020.
- Đorđević, N.; Simić, S.; Ćirić, A. First identification of the cylindrospermopsin (cyn)-producing cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju in Serbia. Fresenius Env. Bull. 2015, 24, 3736–3742. Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29.
- Beasley, V.R. Harmful Algal Blooms (Phycotoxins). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020. Zegura, B.; Straser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41.
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. Flores, M.; Goodrich, D.W. Retinoblastoma Protein Paralogs and Tumor Suppression. Front. Genet. 2022, 13, 818719.
- Zegura, B.; Straser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. 2011, 727, 16–41. Štern, A.; Rotter, A.; Novak, M.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial pentapeptide nodularin in HepG2 cells. Food Chem. Toxicol. 2019, 124, 349–358.
- Flores, M.; Goodrich, D.W. Retinoblastoma Protein Paralogs and Tumor Suppression. Front. Genet. 2022, 13, 818719. Sivonen, K. Cyanobacterial toxins and toxin production. Phycologia 1996, 190, 267–275.
- Štern, A.; Rotter, A.; Novak, M.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial pentapeptide nodularin in HepG2 cells. Food Chem. Toxicol. 2019, 124, 349–358. Harding, W.R.; Rowe, N.; Wessels, J.C.; Beattie, K.A.; Codd, G.A. Death of a dog attributed to the cyanobacterial (blue-green algal) hepatotoxin nodularin in South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 256–259.
- Sivonen, K. Cyanobacterial toxins and toxin production. Phycologia 1996, 190, 267–275.
- Harding, W.R.; Rowe, N.; Wessels, J.C.; Beattie, K.A.; Codd, G.A. Death of a dog attributed to the cyanobacterial (blue-green algal) hepatotoxin nodularin in South Africa. J. S. Afr. Vet. Assoc. 1995, 66, 256–259.
