2. Plant-Derived Exosome-like Nanoparticle as Biomolecules
Exosomes were discovered in 1983 by Eberhard Trams and Rose Johnstone
[23][24][23,24]. Exosomes are biologically small, nano-sized extracellular vesicles (EVs) with a diameter of 30–150 nm and density of 1.13–1.19 g/mL, secreted naturally by almost all eukaryotic cells
[25][26][27][28][29][30][31][25,26,27,28,29,30,31]. Exosomes are important since these vesicles are similar to cargo-containing molecular components, such as DNA, RNA, lipid, and proteins, which are later released into the extracellular matrix as a form of intercellular communication
[32][33][34][32,33,34]. Several studies have shown that exosomes comprise cytokines, transcription factor receptors, and other bioactive compounds
[35][36][35,36].
The exact mechanism of exosome biogenesis remains controversial; however, they are generally synthesized in the multivesicular endosome compartments of the cell and released when these compartments fuse with the plasma membrane. Therefore, exosomes are also known as membrane-bound or membrane-derived vesicles
[26][37][38][39][26,37,38,39]. Formerly, these exosomes were referred to as “ectosomes”, “shed vesicles”, and “small extracellular vesicles (sEVs)”. To date, however, the term “exosome” has received the most popularity
[40][41][42][40,41,42]. In comparison to micro vesicles, apoptotic bodies, and oncosomes, exosomes are the smallest type of EVs
[32][43][44][45][32,43,44,45].
Research proved EVs, such as exosomes, comprise their own specific cargo. This cargo becomes crucial since it represents the health and/or disease status of the source or parent cells, which has the capacity to alter recipient cells, both neighboring and distantly located ones, by adhering to the cell membrane and releasing its internal cargo. The release of this internal load induces physiological, phenotypic, and functional changes in the recipient cells
[26][46][47][48][49][50][26,46,47,48,49,50]. These findings boost numerous investigations into the role of exosomes as novel biomarkers and potential therapeutic agents, which demonstrate that exosomes, as mediators of cell-to-cell communication, are related to physiological and pathological processes on the smallest scale, particularly between cells that occur in the living system
[51][52][51,52].
Exosomes are divided into two categories: Natural and engineered exosomes. Natural exosomes are animal derived, mostly from mammals, and originate from cells
[53][54][55][56][53,54,55,56] or biofluids
[57][58][59][60][57,58,59,60], and plants as natural resources.
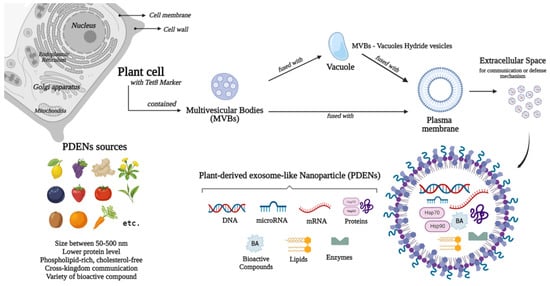
Figure 1 and
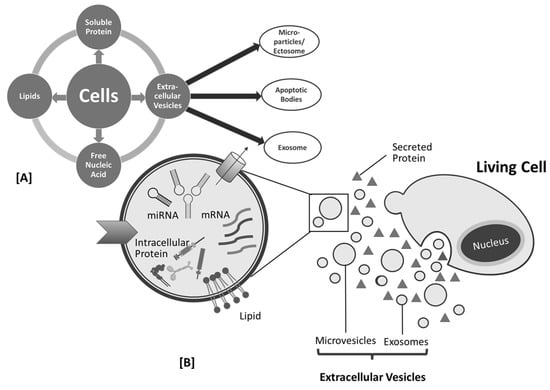
Figure 2 describe the biogenesis, sources, and contents of PDEN- and MSC-derived exosomes, whereas engineered exosomes are natural exosomes that have been artificially modified or loaded with therapeutic agents
[39]. Animal-derived exosomes are further split into normal and tumor exosomes since exosomes can be produced in both normal and tumor conditions
[39]. Studies related to mammalian-derived EVs, including exosomes, are documented in databases, such as Vesiclepedia (
http://www.microvesicles.org, accessed on 17 February 2023) and ExoCarta at
http://www.exocarta.org, (accessed on 17 February 2023)
[61][62][63][61,62,63]. In addition to animals, plants are a source of exosomes, with good development prospects in the future. In recent years, studies have found that nano-sized EVs from plant cells have similar structures to the mammalian exosome
[64][65][66][67][64,65,66,67], which is why these EVs are termed as “plant-derived exosome-like nano-particles” with various abbreviations, such as PDELNs
[68], PLENs
[69], or
plant-derived exosome-like nanoparticles (PDENs
) [70]. Other descriptive terms include “plant-derived nanovesicles (PDNVs)”
[71], “plant-derived extracellular vesicles (PDEVs)”
[72], “plant-derived exosomes”
[73], “plant-derived edible nanoparticles”
[74], or “edible plant-derived nanovesicles”
[75]. Th
is re
following contentsview uses the term “plant-derived exosome nanoparticles”, which is abbreviated as PDENs.
Figure 1.
Sources, biogenesis, and contents of plant-derived exosome-like nanoparticles (PDENs).
Figure 2.
Cell products (
A
) and biogenesis of mesenchymal stem cell (MSC)-derived exosome (
B
).
Plant-derived exosome-like nanoparticles are still not as popular as mammalian-derived exosomes, which have been extensively investigated, but the research trend has been increasing
[71][75][76][77][71,75,76,77]. This positive trend is driven by the idea that consumption of certain foods or their associated components is often linked to health benefits and disease risk reduction
[75]. Furthermore, concerns have been raised regarding the utilization of human exosomes for therapeutic chemical delivery since evidence stated that human exosomes may constitute one of our body’s scavenging processes, restricting large-scale production, and often carrying potentially transferable harmful substances, such as tumor-derived molecules and foreign nucleic acid
[78][79][80][81][82][83][84][78,79,80,81,82,83,84]. Similar to other allograft materials, the large-scale manufacturing and usage of exosomes derived from humans will face obstacles in the future due to exosome sources and ethical concerns.
In addition to plant-derived micronutrients, sterols, fibers, and phytochemicals, studies on plant-derived bioactive compounds and their impact on health have recently been broadened to include EVs
[75]. The Food and Agriculture Organization (FAO) division of International Network of Food Data Systems (INFOODS) collects data on foods containing exosomes (or endosomal-derived vesicles) in their databases;
https://www.fao.org/infoods (accessed on 17 February 2023). The database contains the following information regarding the plant-derived exosomes: Common name, group, scientific name, sample type, isolation method, and references
[72][85][72,85]. PDENs, similar to mammalian-derived exosomes, comprise a variety of components, such as proteins, lipids, mRNA, microRNA, and their unique source-dependent bioactive constituents
[86][87][88][86,87,88]. PDENs are composed of cytosolic and membrane proteins. They contain numerous types of confirmed proteins, such as heat shock proteins, which are accepted as exosome markers (e.g., CD9 and CD63), transmembrane proteins (e.g., actin, proteolysis, aquaporin, and chloride channels proteins), defense proteins, and other plasmalemma-associated proteins
[89][90][91][92][93][89,90,91,92,93]. Research substantiated that PDENs from citrus fruits comprise proteins involved in diverse activities, including fructose bisphosphate for glycolysis, HSP80 and PTL39 for protein folding and transport, and PTL3 and clathrin-3 for cell growth and division. In addition, enzymes and antioxidants were identified
[94]. In terms of protein level, PDENs are lower than mammalian exosomes
[89].
Lipids in EVs can induce cellular responses in recipient cells, maintaining the structural stability of exosomes, facilitating their cargo components (uptake and retention), and playing an important role in intercellular communication
[95][96][95,96]. PDENs are rich in phospholipids (e.g., phosphatidic acids (PA), phosphatidylethanolamines (PE), including plant lipids, such as galactolipids (e.g., monogalactosyldiacylglycerol (MGDG) and di-galactosyl di-acylglycerol (DGDG))
[89][97][89,97]. Based on previous studies, lipid composition approximations of some edible plants have been reported, such as exosome-like nanoparticles derived from ginger, which comprised 40% PA, 30–40% DGDG, and 20% MGDG
[89][95][89,95], turmeric comprised 42% DGDG, 12% MGDG, 20% PA, and phosphatidylcholine (PC) as reported by Liu et al. (2022)
[98], orange juice comprised 40% PE, 25% PC, and 5% PA
[99], and grapefruit comprised 45.52% PE and 28.53% PC
[65]. From the findings, it was known that the amounts of lipid compositions in PDENs vary depending on their sources. Moreover, it was observed that PDENs are different from mammalian exosomes since they are phospholipid-rich but cholesterol-free, whereas animal exosomes are cholesterol-rich
[100][101][100,101].
Plant-derived exosome-like nanoparticles often comprise RNAs and a significant quantity of microRNAs (miRNAs), a class of small and noncoding RNAs (17–24 nucleotides). It has been found that miRNAs are capable of disrupting mRNA translation and transcription
[102][103][104][102,103,104]. Moreover, based on previous studies regarding the potential for cross-kingdom communication by PDENs through gene expression modulation
[105], which involves miRNA, it has been found that the internalization of PELNs is possible in plant cells, mammalian cells, fungi, and bacteria
[101][106][107][108][101,106,107,108]. In addition, several bioactive compounds are found in PDENs, which have the potential to determine their effect. This naturally occurring metabolite varies not only based on plant origin, but also on the EV population size, preparation form, and isolation technique.
Table 1 shows the compounds found in PDENs from several sources. In addition, PDENs comprise myo-inositol, quinic acid, and aucubin, which are anti-cancer and anti-inflammatory compounds
[109].
Table 1.
Metabolites found in PDENs from several sources.
PDENs comprise 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol, which are found in ginger
[89][113][89,113]; naringenin found in grapefruit
[65]; citrate, vitamin C, and galacturonic acid-enriched pectin-type polysaccharide found in lemon
[66][110][66,110]; vitamin C found in strawberry
[88]; epigallocatechin gallate, epicatechin gallate, epicatechin, vitexin, myricetin-3-
O-rhamnoside, kaempferol-3-
O-galactoside, and myricetin found in tea flower
[66][111][66,111]; fiber β-glucan found in oat; and sulforaphane found in broccoli
[112]. In addition, PDENs comprise myo-inositol, quinic acid, and aucubin, which are anti-cancer and anti-inflammatory compounds
[109].
Plant-derived exosome-like nanoparticles need to undergo a series of processes before they can be used. After plant extraction, the first step is isolation and there are several isolation techniques used for PDENs. Most isolation methods are based on density, surface content, size, or precipitation
[114]. Based on density, frequently used techniques are differential ultracentrifugation and density gradient centrifugation. These techniques are simple and cost-effective; therefore, they are considered as the “gold standard” for the isolation method
[74][114][115][116][74,114,115,116]. Differential ultracentrifugation is the consecutive removal of particles based on size and density using progressive centrifugal durations and forces. The procedure is carried out via a step-by-step methodology, starting from the separation of cells, and then gradually cell debris, apoptotic cells, and micro-vesicles. As the remaining particles become smaller, the spinning speed is gradually increased (100,000–200,000 g) throughout the process
[117][118][119][117,118,119]. The formation of sediment vesicles, protein aggregates
[4], and exosomal aggregates
[74][120][74,120], the extended duration, labor intensiveness, and the necessity for highly expensive equipment are the major drawbacks of this procedure
[121][122][123][124][121,122,123,124]. In contrast to differential ultracentrifugation, density gradient ultracentrifugation isolate PDENs are based on size, mass, and density. This method differentiates between PDENs and contaminants with different densities using a subsequent phase with a sucrose density gradient (10–90%) or other material (e.g., iodixanol)
[125][126][125,126]. Furthermore, density gradient ultracentrifugation is still extensively used today since this method increases the final purity of PDENs isolate
[74][117][74,117]. Nevertheless, this technique yields few exosomes, is time-consuming, and requires expensive equipment and specialized knowledge
[127][128][127,128].
Sized-based isolation methods including ultrafiltration and size exclusion chromatography (SEC) are commonly used to separate PDENs. Ultrafiltration, a faster alternative to ultracentrifugation, comprises the use of membrane filters with different molecular weight cutoffs (MWCO) and pressure to remove large molecular size impurities, followed by exosome isolation
[67][129][67,129]. In this method, larger sample components including cells, cell debris, and macromolecules are not permitted to pass through the membrane pores
[39]. Utilizing filters with pore sizes of 0.80 and 0.45 microns, larger particles can be eliminated. After collecting the remaining sample, a filter with pores of smaller diameters than exosomes (0.22–0.1 m) is used to remove particles smaller than exosomes. This approach obtains exosomes between filters with maximum and minimum pore diameters
[130]. Ultrafiltration is simple and efficient without affecting the exosomes activity technique; however, it is low in purity and not suitable for wide usage
[131]. SEC is another size-based method using gel-filtration. The mobile phase is an aqueous solution that transports the sample along the column, while the stationary phase is a porous filtration polymer that permits differential elution. With the support of gravity, larger particles are eluted before smaller ones. However, other factors, such as molecular weight and shape, can also influence this separation process
[21][115][132][21,115,132]. The advantages of this technique include good homogeneity and preservation of the integrity, morphology, and functionality of nanoparticles. However, this technique is not widely used due to low purity production and absence of selectivity in extracting exosomes from particles of almost identical size
[133][134][135][136][133,134,135,136]. Asymmetric flow field-flow fractionation (AF4) is a recent, size-based isolation method. AF4 separates nanoparticles with sizes ranging from a few nanometers to an undetermined amount of micrometers, depending on their hydrodynamic diameters, in a thin, flat channel with a semipermeable wall membrane
[136].
Moreover, the polymer precipitation method can be used to isolate PDENs, although this method is originally used to isolate viruses
[137][138][139][137,138,139]. The scientific reasoning is due to the fact that both viruses and small PDENs have similar biophysical characteristics
[39]. This method uses polyethylene glycol (PEG), specifically PEG6000, which is capable of forming a net-like structure to trap PDENs before precipitation. These precipitated and aggregated PDENs are harvested through lower centrifugation rates
[140]. This technique is easy to operate, time-efficient, and capable of processing large doses of samples
[39]. Furthermore, this study illustrates that this technique is less expensive and outperforms ultracentrifugation in terms of purity and recovery
[141]. Polymer precipitation using concentration-adjusted PEG6000 produced PDENs with a smaller particle size than ultracentrifugation
[142]. However, impurity and low recovery time are the primary disadvantages of this technique
[39][143][39,143].
Another method for isolating PDENs is the affinity-based isolation method or immunoaffinity chromatography (IAC). IAC is a separation and purification method based on the specific interaction of antibodies and ligands to isolate desired components from the heterogenous mixture
[39][115][39,115]. The biomarker or antigen should be a high-abundance protein on the surface of exosome membranes, such as the four-transmembrane protein superfamily and ESCRT complex-related proteins
[39][143][39,143]. Several methods have been proven to be successful for isolation-related IAC, among these are magnetic beads
[144] or FeO
3 nano-cubes
[145] loaded or coated with antibodies, as well as heat shock proteins
[146], heparin
[147], or epithelial cell adhesion molecules
[148]. These methods accommodate high-purity exosomes and selectively isolate exosomes that carry a specific marker
[114]. Nevertheless, the drawbacks of these methods include the heterogenous nature of PDENs and the loss of exosomes integrity by removal of the antibody process
[149][150][149,150]. In addition to the aforementioned common methods, few other advanced methods can be used to isolate the PDENs, such as microfluidics-based isolation by trapping PDENs in a porous microstructure with a selective micropillar design of 40–100 nm in diameter
[151]. This can be performed via the acoustics-based approach using a combination of microfluidic chip and acoustic waves
[152], or a commercially available exosomes isolation kit that is time-saving but costly, such as total Exosome Isolation kit (Invitrogen), exoEasy Maxi kit (QIAGEN), MagCapture™ Exosome Isolation Kit PS (Wako), Eloquence (System Biosciences), Exo-spin (Cell guidance systems), and Minute™ Hi-Efficiency Exosome Precipitation Reagent
[39][153][39,153]. These kits can be used to separate exosomes from biological samples, such as serum, plasma, and CSF. However, the purity, amount, and size distribution of the collected exosomes are very different
[154][155][154,155].
Plant-derived exosome-like nanoparticles, such as mammalian exosomes, can be characterized using the ultrasensitive microscopic technique, such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), or using cryo-electron microscopy for structural analysis in subcellular level
[119][156][119,156]. Optical characterization approaches cannot identify PDENs due to their small diameters; therefore, various methods of electron microscopy are essential to determine their morphological size and shape
[157]. SEM can be used to observe the surface structure; however, it is lower in resolution. Meanwhile, the high-resolution TEM provides information regarding the internal structure and particles distribution; however, it is not suitable for rapid measurement since the pre-processing step is more complex
[158][159][158,159]. Sample preparation for TEM proved to be responsible for the observed morphology variations
[160][161][160,161].
Particles charge and size of PDENs can be observed using dynamic light scattering (DLS); however, this technique cannot detect the concentration and is not suitable for measuring complex exosomes with large size range. The concentration of PDENs can be measured using the nanoparticle tracking analysis (NTA)
[39][119][39,119]. This technology emerged as the “gold standard” for exosomes characterization since PDENs can be observed in real-time due to their fast detection speed; however, the operation is complex and can affect the PDENs quantification
[39][135][162][39,135,162]. To determine the size and concentration of PDENs in suspension, resistive pulse sensing (RPS) technology can be used. Since a voltage is applied, the detection of exosomes by RPS with the transfer of single nanoparticles through the nanopore is established
[156][163][156,163]. Western blot and enzyme-linked immunosorbent analysis (ELISA) can be used to detect the expression of PDENs marker proteins
[41][164][41,164]. Flow cytometry can be used to detect the biomarker of PDENs. Flow cytometry is a high-throughput technology and capable of high-speed multi-channel analysis with low sample concentration
[39][156][39,156]. However, the newest generations of digital flow cytometry instruments, with a lower limit of measurement of 100 nm, are still unreliable in terms of accuracy and resolution
[156]. Another technique to characterize PDENs specifically for the determination of the PDENs weight can be performed using the developed nanomechanical resonator. Moreover, zeta analyzer can be used to observe the repulsive nature against aggregation or dispersity and the membrane potential
[119][165][119,165].
Similar to the most common exosomes, PDENs cannot be maintained for a long period of time. Therefore, it is necessary to preserve PDENs in order to maintain their biological activities and for readily accessible clinical application. Plant extracts stored prior to PDENs isolation may influence the separation, content, and function of PDENs, such as those reported in EVs derived from biofluids. Before the isolation process, samples were mainly stored in freezing conditions for short or long periods. Although 4 and −80 °C or lower are mentioned and generally used in laboratories today, the problem is that no optimal storage condition has been determined for isolated EVs
[166][167][168][169][170][171][166,167,168,169,170,171]. EVs may alter to varying degrees during storage, resulting in size, shape, function, and content loss
[172]. Current techniques to accommodate this include cryopreservation, freeze-drying, and spray-drying
[39][173][39,173].
Cryopreservation is the application of low temperatures below those needed for biochemical reactions (4 °C, −80 °C, or −196 °C) to preserve the functional stability of biological particles, including PDENs, using cryoprotectants
[166][172][173][174][166,172,173,174]. There are two types of cryoprotectants which are differentiated based on their permeability or penetration and both aimed to reduce the injury of EVs. Penetrating cryoprotectants (e.g., glycerol, DMSO, and ethylene glycol) have a small molecular weight that can penetrate the cell membrane, while non-penetrating cryoprotectants (e.g., sucrose, mannose, and trehalose) form a hydrogen bond with water to prevent ice crystal formation
[159][175][176][159,175,176]. A previous study has proven that cryopreservation in −80 °C with or without cryoprotectants, such as trehalose 25 mM, DMSO 6 and 10%, and glycerol 30%, can maintain the concentration of EVs up to 6 months
[176]. However, storage conditions may vary depending on the source of EVs. Regarding safety, trehalose as a non-penetrating type, is the best option and most effective antifreeze
[175][177][175,177].
Freeze-drying process is a technology that involves sublimation and desorption, where moisture-containing materials are frozen to a solid below the freezing point prior to their use
[39][172][39,172]. Freeze-drying is a new technique for preserving EVs, including PDENs, and the optimal storage temperature for freeze-dried EVs is 4 °C
[178][179][178,179]. Freeze-drying is advantageous in the expansion of the lifespan of heat-sensitive materials, such as EVs, vaccines, and proteins since it can maintain the material’s original activity, while causing less damage to biological tissues and cell bodies. Moreover, the material can be easily stored in a constant state and reconstituted by simply adding water. However, due to the freezing and dehydration pressures generated during the freeze-drying process, the molecular structure of the biomolecule may be destroyed
[39][172][39,172]. Based on previous studies, cryoprotectant products, such as trehalose, may be used to prevent aggregation during the freeze-drying process and reduce the alteration in the stability and morphology of EVs, including PDENs
[179][180][181][179,180,181]. Spray-drying, on the other hand, is an option. Spray-drying is a one-step method that is simpler than freeze-drying
[172]. This technique is more economical, can be used with a variety of agents, and allows for product size adjustment. First, PDENs solution is atomized in a drying room, and then when moisture interacts with hot air, it quickly vaporizes, resulting in dry powders
[39][174][39,174]. PDENs stability can be affected by factors, such as EV solution feed, atomization pressure, and output temperature. Furthermore, residual moisture may cause chemical instability by lowering the solid particle state’s glass transition temperature
[172].
The advantages of PDENs as biomolecules are: (1) Provided by nature and can be involved in intercellular communications, (2) phospholipid-rich characteristics in order that the lipid membrane can protect the cargo from the external agent, which can deteriorate the bioactive compound inside the PDENs, (3) natural mechanism of cellular uptake in order that they may have the ability to pass through some of the barriers in our body, such as blood brain barrier and the placenta, (4) tolerated by the immune system as they are currently present in foods ingested by humans, (5) verified scalability and suitability for industrial applications, (6) non-toxic, as they are produced from organic fruits and vegetables
[71], and (7) long-term availability from numerous types of plants, which can be cultivated. These PDENs are worth development in various fields, especially in the field of biomolecular medicine. This can be accomplished by exploring the cargo, in particular, the contents and functions of bioactive constituents. Then, they can be related to the necessary various problem-solving strategies. A better understanding of PDENs in the future has the potential to usher in a new paradigm for natural medicine, which employs compounds that are abundantly available, more effective, efficient, and have significantly fewer adverse effects than the currently accessible medications.
Exosomes have been investigated in animal models and clinical trials in recent years to obtain their biological properties. Grape-derived PDENs have been proven to be nontoxic to intestinal macrophages, splenic, and liver cells of mouse model
[65][100][65,100]. Ginger-derived PDENs are nontoxic to colon-26 epithelial-like cell lines, and RAW 264.7 macrophage-like cell lines in the colon’s mouse model
[89]. Another study proved that citrus lemon-derived PDENs inhibit the growth of CML tumors in vivo by reaching the tumor site and activating TRAIL-mediated apoptotic cell processes in the liver, spleen, and kidney of mouse model
[182]. Moreover, ginseng-derived PDENs proved to be nontoxic to BMDMs (bone marrow-derived macrophages), B16F10 (mouse melanoma cell line), 4T1 (mouse mammary carcinoma line), and HEK293T (human embryonic kidney cell line) in the liver and spleen of mouse model
[81]. The development of human exosomes and PDENs as potential biomolecules has reached the clinical trial stage
[183][184][183,184]. According to a ClinicalTrials.gov survey (
https://clinicaltrials.gov/, accessed on 17 February 2023), exosomes are used for clinical trial as biomarkers (50%), exosome-therapy (28.44%), drug delivery systems (5.17%), cancer vaccines (1.72%), and other analysis (14.66%)
[183]. Two reported clinical trials using PDENs aimed to encapsulate curcumin into grape-derived (NCT01294072) PDENs and ginger-derived (NCT04879810) PDENs for the treatment of colon cancer and irritable bowel disease, respectively. Another clinical trial using grape-derived PDENs (NCT01668849) aimed to investigate the ability of grape-derived PDENs to prevent oral mucositis associated with head and neck cancer chemoradiation treatment. However, none of these trials have been completed, and the results are not yet available
[183][184][183,184]. Furthermore, although several experimental studies have been documented, there is less evidence on GMP manufacturing for plant-derived exosomes
[21][185][21,185].