1. Introduction
The metallurgy process for metal refinement frequently requires energy-intensive melting steps based on the use of fossil fuel. Pyrometallurgy, hydrometallurgy and biohydrometallurgy are three variants of metallurgy that differ in the mechanism of extraction. While pyrometallurgy uses heat power, hydrometallurgy applies redox chemical reactions in an aqueous or organic liquid solution. Biohydrometallurgy works similarly to hydrometallurgical processes, except that the reagents are directly supplied by the microorganisms because of the by-products of their metabolic reactions.
Biohydrometallurgy operates similarly to hydrometallurgical processes, except that the chemicals are supplied directly by microorganisms as byproducts of their metabolic reactions. Research is rapidly evolving to manage the challenges in recycling e-waste for metal recovery through both chemical and biological routes. As part of the chemical method, hydrometallurgy is a well-established process for metal leaching from both primary and secondary resources
[1][2][19,20]. Conversely, biohydrometallurgy is still under investigation in several aspects, such as the physiology of less explored microorganisms, bioprocess operation and scalability
[3][4][5][21,22,23].
The biological route is carried out by specialised leaching microorganisms which implement strategies for recovering metals against their scarcity in the environment.
The microbial systems acquire metals necessary
required for metabolism and counteract the adverse effects of toxic metals to protect the cell by using a whole repertoire of mechanisms and
in order to acclimatise themselves to hostile environmental conditions
[6][24]. Microbial growth, metabolism and differentiation are intimately linked to the biogeochemical cycle of metals. The Metals can be classified into three categories based on their different physiological roles: (a) vital and non-toxic, such as Ca and Mg; (b) vital but toxic at elevated levels, such as Fe, Mn, Zn, Cu, Co, Ni, and Mo; and (c) toxic, such as Hg and Cd
[7][8][25,26]. Metals such as aluminium (Al), antinomy (Sb), arsenic (As), barium (Ba), beryllium (Be), bismuth (Bi), cadmium (Cd), gallium (Ga), germanium (Ge), gold (Au), indium (In), lead (Pb), lithium (Li), mercury (Hg), nickel (Ni), platinum (Pt), silver (Ag), strontium (Sr), tellurium (Te), thallium (Tl), tin (Sn), titanium (Ti), vanadium (V), and uranium (U) have no established biological functions and are considered as non-essential metals
[6][24]. Nevertheless, there is evidence of microbial removal of some of these non-essential metals
[3][4][5][9][21,22,23,27].
Natural sources of metal-leaching microorganisms are mine sites or acid mine drainage samples, though other sources exist (e.g., brines and sediments, sludge from anaerobic digestion plants)
[10][28]. Due to the physiology of these microorganisms, bioleaching can be economically feasible (e.g., in situ processing is viable thanks to the simplicity of technology design and operating conditions; the possibility of reusing existing facilities; and reasonable capital and operating costs) and eco-friendly approach with higher efficacy, safety and ease of control
[11][29]. Furthermore, the waste streams, including toxic gas emissions and wastewater, are more restricted and controlled in the context of microbial leaching. However, reaction kinetics can be pointed out as the main limitation of biohydrometallurgy processes, being strictly dependent on the characteristics of the biocatalyst.
Factors affecting the bioleaching process include pH, temperature, oxygen, and carbon dioxide supply, as well as nutrients in the medium. pH is selective only for certain metal compounds (e.g., carbonates, common oxides, acid-soluble sulphides), and of course, it is also a filter for microbial growth and activity
[12][13][14][30,31,32].
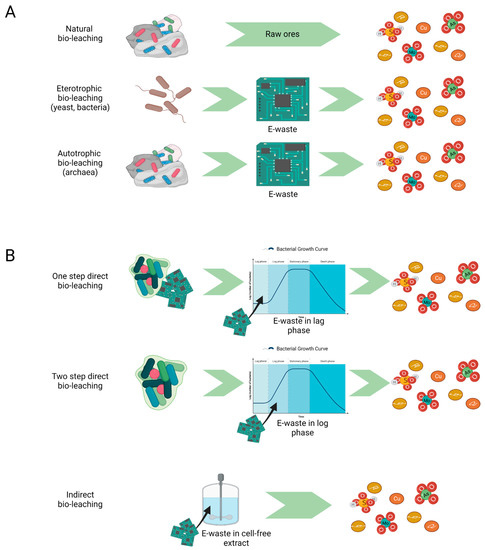
Bioleaching essentially occurs via autotrophic and heterotrophic mechanisms
pathways, with the former being either direct or indirect, whilst the latter being only
purerly indirect
[9][13][27,31], as depicted in
Figure 1.
Figure 1. Bioleaching: mechanisms and strategies. (A) Heterotrophic and autotrophic bioleaching occurring in natural minerals deposits and in e-waste recovery processes resulting in free metal ions and metal oxides; (B) Direct (one-step and two-step) and indirect approach to bioleaching process. In direct bioleaching, e-waste is introduced during the initial microbial growth phase (one-step) or exponential growth phase (two-step). In indirect bioleaching, only microbial metabolites interact with e-waste.
Direct bioleaching implies the contact between microorganisms and metallic materials. This is valid for both single- and double-step processes, where the solid containing metals are mixed with microorganisms from the beginning or during the exponential microbial growth, respectively. Though direct and indirect bioleaching are both effective methods, the first one may run into the inhibition of microbial activity due to the toxicity of leached metals
[13][31]. However, direct contact promotes the adhesion between microorganisms and the solid surface, mediated by the formation of biofilms and the uptake or complexation of metals through the excretion of bio-lixiviants and chemicals that perturb the environmental state, respectively, ending with the release of metal ions into solution. Recently, insights into the mechanism of interspecies communication within the archaeal biofilm and its regulation have enabled a deeper understanding of the bioleaching processes
[15][33]. Autotrophic bioleaching uses carbon dioxide as a carbon source and iron Fe (II) or sulphur S (0) as the main energy sources to carry out their oxidation
[16][34]. The general chemical reactions occurring in bioleaching are extensively reported in several studies
[11][17][18][29,35,36] and more recently by Magoda K. and Mekuto L. (2022)
[19][37]. Sulfur-oxidising bacteria (e.g.,
Acidithiobacillus thiooxidans,), iron- and sulphur-oxidising bacteria (
Acidithiobacillus ferrooxidans) and iron-oxidising bacteria (
Leptospirillum ferrooxidans) are the most employed autotrophs in bioleaching processes because of their resistance to heavy metal toxicity and their simple nutritional requirements
[20][38]. .The consequent acidification due to the production of sulfuric acid and ferric ions causes metal dissolution, although for acid-insoluble sulphides or some metal oxides can be recovered either through biological oxidation, when the metal is soluble at a high oxidation state, or biological reduction, occurring when the metal is more soluble in a low oxidation state (e. g., Ni, V, and Mn)
[21][39]. In contrast, heterotrophic bioleaching occurs via an indirect mechanism based on organic acids (e.g., citric or oxalic acid), ligands (e.g., chelators and siderophores) and exopolysaccharides (EPS) biosurfactants
[15][33]. Among archaea,
HaloferaxHaloferax mediterranei mediterranei has been chosen as a model organism for EPS production, achieving a technological level of readiness (TRL) of 2
[22][40]. Heterotrophic bioleaching can be performed via acidolysis (
AcidothiobacillusAcidothiobacillus ferroxidans ferroxidan
s and
Leptospirillum ferroxidans) or complexolysis
(Chromobacterium violaceum, Pseudomonas aeruginosa, Pseudomonas flourescens, and Bascillus megaterium). In acidolysis, organic acids produce complexes with metal ions to enhance their mobilisation through a protonation mechanism, while complexolysis occurs when a chelating agent combines with metal on the surface or via biosurfactants acting as complexing agents. A third possible mechanism for heterotrophic bioleaching is the Fe (III) or (Mn (IV) dissimilatory reduction under anaerobic conditions in complex media, where the oxidation of organic matter is coupled to the metal reduction
ShewenellaShewenella putrfaciens putrfaci
ens is s a reference microorganism for this process
[23][41].
Bioleaching has been successfully applied in indirect and direct approaches for the recovering of valuable metals (i.e., Ni, Si, Cu, Ga, Mg, Te, Zn) from the dismissed light emitting diode (LED)
[9][27], end-of-life PV solar cells
[24][42], printed circuit board (PCB)
[25][4] and other e-waste
[1][26][27][28][19,43,44,45].
2. Interactions between Archaea and Metals
Microorganisms belonging to the Archaea domain are widely recognised as cosmopolitan
ubiquitous organisms capable of adapting to either natural or anthropogenic environments characterised by extreme life conditions (i.e., natural gas and oil reservoirs, acid mines, hydrothermal vents)
[29][30][31][32][33][46,47,48,49,50]. The relation between metals and thermophilic archaea stems from their natural ability to live at high temperatures and high-metals concentrations
[7][25], thanks to unique cell wall structures, thermostable enzymes, and metabolic features
[34][51].
Archaea also requires metals because of their role in enzymatic structures and co-factors, as final electron acceptors, or for sustaining their growth with metal enzymes and for pathways requiring metal ions as co-factor constituting the archaeal metallosome
[31][48].
Genomic and proteomic studies allowed for the identification of similarities between archaeal, bacterial and eukaryotic metallosome, with the most common domains coding for Fe-, Co-, and Zn- binding proteins and only a low percentage coding for binding domains (<0.3%) for Ni-, Cu-, and Mo-
[35][52]. Nonetheless, a higher percentage of Fe- binding domains were identified in archaeal genomes (≈7.1%) when compared to bacterial (≈3.9%) and eukaryotic (1.1%) ones. Although less represented, metal binding domains related to Ni-, Co- and Cu- utilisation/transport were also identified in archaeal genomes
[35][36][52,53].
Table 1 resumes the roles that the previously mentioned metals have in Archaea.
Table 1.
Physiological role of essential metals in some selected species of Archaea.
| Metal |
Microorganism |
Function in Archaea |
References |
| Fe- |
Halobacterium spp., Methanosarcina spp., Methanobacterium spp., Sulfolobus spp., Thermoplasma spp., Ferroplasma spp., Pyrobaculum spp. |
Fe (II)oxidation, Fe (III) reduction, Fe4S4-ferredoxin, Fe4S4 cluster for S- adenosylmethionine cleavage, Ni-Fe hydrogenase |
[31][35] | 52 | [37] | ,54 | [38] | ,55 | [39] | [48,,56] |
| Zn- |
n.s. |
“Small proteins” class genes (Zn finger motifs and Really Interesting gene (RING)) |
[35] | [52] |
| Co- |
Methanosarcina spp., Sulfolobus solfataricus, Thermoplasma acidophilum |
Found in co-enzyme B12 structure, Ni/Co uptake system |
[40][41] | [57,58] |
| Ni- |
Sulfolobus spp., Halobacter spp., Methanococcus spp. |
Enzymatic co-factor for different enzymes:Ni-Fe hydrogenase, CO de-hydrogenase, methyl-CoM reductase, urease |
[39][40][41] | [56,57,58] |
| Cu- |
Halobacterium spp., Methanosarcina spp., Methanobacterium spp. |
Copper-binding proteins, N2O reductase |
[37] | [54] |
| Mo- |
Sulfolobales spp., Halobacteriales spp., Methanosarcinales spp., Methanococcales spp., Methanomicrobiales spp. |
Molybdenum co-factor (Moco) involved in W utilization |
[42] | [59] |
With archaeal life requiring metals that are classified as CRMs (i.e., Co) and toxic materials (i.e., Ni, Co, Mo) and being capable of storing them in cellular structures (i.e., enzymes, co-factors, proteins) applications of archaeal cultures for metal detoxification and recovery surely sounds appealing. Furthermore, different studies have highlighted how archaeal-driven biotechnological applications (i.e., biomethanation, anaerobic digestion) need a stable supply of trace metals, including Fe, Co, Ni, Cu, and Mo, in order to maintain process stability
[43][44][45][46][47][60,61,62,63,64].
A possible solution for economically viable metal recovery could be represented by its integration within already operating processes. In this logic, the modulation and fine-tuning of waste metals, supplied as nutrients, could sustain the systems requirements while increasing the sustainability of the process through the integration and valorisation of different waste streams.
Although most knowledge about the interaction of archaea with metals focuses on the role of Fe, Co, Ni, Cu, and Mo, many sources have reported interactions with non-essential metals, also listed as CRMs, precious and toxic elements (i.e., As, Cd, Pt, V, and U)
[3][4][5][9][21,22,23,27]. A deeper insight regarding such archaea-metal interaction will be provided in further sections.
3. Bioleaching among Archaea
Despite the peculiar ubiquities and the great biotechnological potential of Archaea, Bacteria and Eukarya have always dominated the scene in terms of research studies, industrial application, and public perception.
Despite Archaea's unusual ubiquity and tremendous biotechnological promise, Bacteria and Eukarya have traditionally dominated the scene in terms of study, industrial use, and popular perception. As detailed in the review authored by
[22][40] on the current status of archaeal cell factories in bioproduction, few companies are investing in Archaea, while commercialisation is limited to extremophilic genera, which are suitable for applications in industrial processes under harsh conditions
[48][65]. As regards the biomining and bioleaching industry, the engagement of Archaea is very limited, while research studies are mainly restricted to thermoacidophiles genera, often cultured with bacterial species whose leaching ability has already been validated
[7][49][25,66]. However, archaea are also members of non-extreme environments
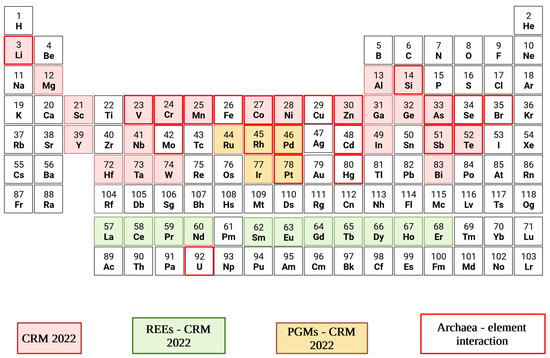
[50][67]. Thus, the scenarios of possible industrial applications and their operating conditions are multivariate. A panoramic of CRMs typically contained in e-wastes and targeted by the Archaea herein discussed is presented in
Figure 2.
Figure 2. Periodic table of elements categorised as Critical Raw Materials in 2022, comprising PGMs and REEs
[51][52]as listed in Table S1 [13,14]. The red squares represent archaea-element interactions described in the
prese
archnt work.
3.1. Extremophile Archaeon
Several species of thermoacidophiles Archaea, such as
Acidianus spAcidianus sp, Ferroplasma acidophilum,
Ferroplasma acidophilumMetallosphaera sp,
Metallosphaera spMetallosphaera sedula,
Metallospha
era sedula, have been identified in stirred-tank mineral bioleaching and bio-oxidation operations
[53][68]. Other species isolated from a hydrothermal pool and mineral sulphide ores, respectively
AcidiplasmaAcidiplasma aeolicum a
eolicum and
AcidiplasmaAcidiplasma cupricumulans cupricumulans [54][69], can oxidise and reduce iron. The Sulfolobacaee family comprises species representative of acid hot spiring able to oxidise elemental sulphur (i.e.,
SulfolobusSulfolobus acidocaldarius DSM 639, Sulfolobus solfataricus P2, Sulfolobus tokodaii JCM 10545, Stygiolobus azoricus DSM 6296) acidocaldarius DSM 639[70,
Sulfolobus solfataricus P2, Sulfolobus tokodaii JCM 10545, Stygiolobus azoricus DSM 6296) [55][56]71]. Among
Thermoplasmatales,
reswe
archers can mention
PicrophilusPicrophilus torridus torridus and
ThermogymnomonasThermogymnomonas acidicola JCM 13583, acidicola JCM 13583, which inhabit dry solfataric fields
[57][58][72,73],
ThermoplasmaThermoplasma acidophilum DSM 1728 a
cidophilum DSM 1728 and
ThermoplasmaThermoplasma volcanium GSS1 volcanium GSS1 [59][74], respectively identified from coal refuse piles and hot acid springs. Novel species isolated from acid mine drainage (AMD), such as
Cuniculiplasma divulgatum JCM 30642,
CandidatusCandidatus Micrarchaeum acidiphilum ARMAN-2 NIA Candidatus Parvarchaeum acidiphilum ARMAN-4 NIA Candidatus Parvarchaeum acidiphilum ARMAN-5 Micrar
chaeum acidiphilum ARMAN-2 NIA Candidatus Parvarchaeum acidiphilum ARMAN-4 NIA Candidatus Parvarchaeum acidiphilum ARMAN-5 are lie likely capable of oxidising metal sulphides
[12][30].
Heavy metals, such as As, Hg, and Cd, can be remediated by several extremophilic archaeon species
[21][39].
SulfolobusSulfolobus acidocaldarius strain BC acidocaldarius strain BC was isolated in an acidic, sulfuric thermal spring in the Yellowstone National Park and recognised to be capable of oxidising arsenite As (IV) to arsenate As (V)
[60][75]. Other Archaea strains, such as
Aeropyrum pernix K1, Pyrobaculum calidifontis JCM 11548,Aeropyrum pernix K1, Pyrobaculum calidifontis JCM 11548, and Sulfolobus tokodaii 7 and
Sulfolobus t
okodaii 7 and the genus
Halorubrum contain arsenite oxidase genes
[61][62][63][76,77,78].
SulfolobusSulfolobus solfataricus s
olfataricus species,
Halococcus,
Halobacterium, and, to a lesser extent,
Haloferax genera were capable of mercury Hg (II) volatilisation into Hg (0), thanks to the presence of mercury reductase genes
[64][65][66][79,80,81].
HalobacteriumHalobacterium noricense noricens
e stands out for its ability to adsorb cadmium
[67][82], while
HaloferaxHaloferax strain BBK2 strain BBK2 uptakes cadmium intracellularly
[68][83]. Among metalloids included in the CRMs list, Si is of primary importance given its large application in PV solar cells
Si is crucial due to its widespread use in PV solar cells.. The hyperthermophilic sulphur-metabolising
PyrococcusPyrococcus abyssi abyssi was fossilised during and after exposure to a silica-saturated solution (about 500 ppm of SiO
2) in a simulated hydrothermal environment
[69][84], demonstrating the ability to bind silica at the S-layer sites and integrate it in replacement of the cell wall.
Based on the above-mentioned studies, thermophilic and hyperthermophilic archaea can be applied in many types of high-temperature metal-contaminated waste streams, while haloarchaea can be used in the treatment of hypersaline environments and wastewaters for heavy metal removal.
3.2. Methanogenic Archaea
Methanogens, which already play an important role in the global methane (CH4) cycle by producing CH4 via methanogenesis, can also recover many critical, platinum group metal (PGM) and non-critical metals from the —end-life products, mining residues and waste streams.
Consortia
It has been stated in many studies that anaerobic granular sludge is efficient in PGM metals recovery (
Figure 2). The work by Espadas et al. reports Pd (II) removal via reduction to Pd (0) nanoparticles or biosorption into the biomass of a methanogenic granular sludge from a full-scale up-flow anaerobic sludge blanket (UASB) reactor processing brewery wastewater
[70][85]. While the reduction route was supported using external H
2, formate, and ethanol, the biosorption dominated when acetate, lactate and pyruvate were added as external electron donors. A more recent study determined the ability to reduce Pt (II) and Pt (IV) to Pt (0) with the same anaerobic sludge applied by Pat-Espadas et al. 2018
[70][85]. Rh (III), which is one of the rarest elements in the geosphere (about 0.001 ppm), was also reduced to Rh (0) nanoparticles, with ethanol as an external donor of electrons
[71][86]. Ramos-Ruiz et al., 2016 highlighted the potential of anaerobic granular sludge from a methanogenic reactor as a biocatalyst for the reduction of both Te (IV) and Te (VI) to produce Te (0) nanoparticles
[72][87].
Methanobacterium genus was detected as predominant among the archaeal community populating a fluidised bed reactor applied to lithium-ion batteries (LIBs) leaching via biogenic hydrogen sulphide
[73][88].
Pure Culture in H2/CO2
Compared to consortia, pure culture applications present some advantages in terms of process reproducibility and simplicity. However, while pure cultures usually require more strict conditions (e.g., nutrient demand and key process parameters), consortia are typically more robust
[43][60]. The mesophilic hydrogenotrophic strain
MethanobacteriumMethanobacterium bryatii bryatii BKYH has been isolated from a copper mining area in the Upper Peninsula of Michigan and is able to chelate Cu by secreting a specific protein in response to copper exposure
[74][89]. Increased Cu concentration in the medium results in the production of an extracellular Cu response- (CRX) protein encoded by the
crx gene and promoted by an archaeal Cu-responsive promoter, which could be synthesised as part of a more generic stress response
[75][90].
Zhang et al., 2014 published the first study reporting methanogenic archaeal strains as capable of reducing vanadium (V)
[76][91], classified as CRM in 2022 (
Table S1; Figure 2).
MethanosarcinaMethanosarcina mazei ma
zei and
MethanothermobacterMethanothermobacter thermautotrophicus thermautotrophic
us could reduce up to 10 mM and 5 mM of vanadate V(V) to vanadyl V(IV), respectively, inducing solid extracellular precipitation as the bioreduction occurred at the cell membrane level. However, at some point, methanogenesis stops with vanadyl generation, possibly due to the redirection of electrons from methanogenesis to vanadate reduction.
According to Singh et al., 2016
MethanothermobacterMethanothermobacter thermautotrophicus thermautotrophi
cus is s capable of reducing Co (III) present at a maximum concentration of 4 mM
[77][92] and can reduce up to 1 mM of Cr (VI) to Cr (III) without an inhibitory effect on methanogenesis and cell growth through an intracellular and extracellular reduction mechanism
[78][93]. Holmes et al. reported the potential role of acetoclastic methanogens belonging to the genus
Methanosarcina in reducing U(VI) present in contaminated groundwater
[79][94]. After a period of acetate amendment in the field, the predominance of
Methanosarcina corresponded to methane accumulation and U(VI) reduction. Like
PyrococcusPyrococcus abyssi, abyssi, the hyperthermophilic, hydrogenotrophic methanogen
MethanocaldococcusMethanocaldococcus jannaschii jannaschii could bind Si on its cell wall and accomplish the silicification mechanism, but only when metal cations, particularly Fe (III), were present
[80][95]. However, a more detailed comprehension of this mechanism is pivotal in order to understand its exploitability in the recovery of Si-rich wastes.
3.3. Adverse Interactions between Methanogens and Critical Metals
Despite both methanogenic consortia and pure cultures have been proven capable of reducing different metals, studies have also reported potential issues related to the metal-methanogen interactions as:
-
Inhibition and toxicity: 50% inhibiting concentrations (IC50) for Cd, selenite, tellurite, and tellurate have been investigated on methanogenic consortia, with acetoclastic methanogens displaying higher IC50 (IC50
Cd 8.6 mg/L, IC50selenite 24.1 mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) than those of hydrogenotrophic methanogens (IC50Cd 2.9 mg/L, IC50selenite 18 mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) [81]Cd 8.
6 Similarlymg/L, IC50
for Pd(II), Pt(II) and Pt(IV) for methanogens were reported at 2.7, 2.4 and 3.7 mg/L, respectively [70],selenite whilst24.1 full inhibition of methanogenesis occurred at Pt(II) concentration higher than 5 mg/L [82];mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) than those of hydrogenotrophic methanogens (IC50Cd 2.9 mg/L, IC50selenite 18 mg/L, IC50tellurite 8.6 mg/L, IC50tellurate 10.2 mg/L) [96]. Similarly, IC50 for Pd(II), Pt(II) and Pt(IV) for methanogens were reported at 2.7, 2.4 and 3.7 mg/L, respectively [85], whilst full inhibition of methanogenesis occurred at Pt(II) concentration higher than 5 mg/L [97];
-
Competition between metal reduction and methanogenesis: Methanogenesis, both in anaerobic digestion and in the biomethanation process, requires electron donors to reduce CO
2. When considering the bioleaching process, the addition of an external electron donor is often needed due to the competition for electrons that are redirected from methanogenesis to metal reduction [76]2.
IWhen
an anaerobic granular sludge, endogenous substrates can provide sufficientconsidering the bioleaching process, the addition of an external electron
equivalents for the leaching of metals of interest, although reduction rates may increase with excess electrons, as reported by [72]donor is often needed due to the competition for electrons that are redirected from methanogenesis to metal reduction [91].
AmoIn
g external electron donors to be added to methanogenic anaerobic an anaerobic granular sludge, e
thanol is considered a safe and economical option. Fermentation of ethanol by the acetogenic community populating the granular sludge generates H2 that is then redirected to metal ndogenous substrates can provide sufficient electron equivalents for the leaching of metals of interest, although reduction
. However, it should be mentioned that some studies have described the abiotic reduction of some metals through direct chemical reduction by H2 and formate [70][82][83]. rates may increase with excess electrons, as reported by [87]. Among external electron donors to be added to methanogenic anaerobic sludge, ethanol is considered a safe and economical option. Fermentation of ethanol by the acetogenic community populating the granular sludge generates H2 that is then redirected to metal reduction. However, it should be mentioned that some studies have described the abiotic reduction of some metals through direct chemical reduction by H2 and formate [85,97,98].
3.4. Anaerobic Methanotrophic Archaea (ANME)
Microorganisms play an important role in the global CH
4 cycle that is controlled by the balance between anaerobic production via methanogenesis and CH
4 removal via methanotrophic oxidation. Methane oxidation is anaerobic (AOM) in more than 90% of cases
[84][99]. AOM can be coupled to other electron acceptors besides the well-known sulphates SO
42−, such as nitrates NO
3− (N-DAMO), or metals such as manganese Mn (IV), iron Fe (III), As (V), Cr (VI), Se (VI), Sb (V), V (V), and Br (V)
[85][100] through the establishment of a syntrophic partnership as electron sinks. However, it is not fully elucidated whether the reduction of metallic electron acceptors is independent or supported by electron transfer to syntrophic partners, interspecies electron transfer, nanowires or conductive pili
[86][101].
The study by Zhang et al., 2020 investigated the bio-reduction of vanadate V(V) present in groundwater using an anaerobic sludge inoculum taken from a wastewater treatment resource
[87][102]. Biological mediated vanadate removal, corresponding to 95.8 ± 3.1% of 1 mM, occurred after seven days of incubation using CH
4 as the sole electron donor. Microbial community analysis revealed a more significative change in the archaeal population than in the inoculum, with a massive presence of the
Methanobacterium genus, which is known to oxidise methane via reverse methanogenesis, and the hydrogenotrophic methanogens belonging to
Methanomassiliicoccus. Thus, vanadate reduction occurred through the anaerobic oxidation of methane and synergistic interactions with methane-oxidising bacteria such as
Methylomonas. Evidence of the reduction of antimonate Sb (V) to Sb (III) using CH
4 is reported by the work of Lai et al., 2018
[85][100] in a membrane biofilm batch reactor inoculated with a pre-enriched culture with methane and Sb (V),
Figure 2. Increasing concentrations of Sb (V), 0.41, 0.82, 1.6 mM, at different stages of the experiment were 100% biologically reduced in the form of Sb
2O
3 crystal precipitates. The Archaea community was widely enriched during the different operational stages, where
Methanosarcina and
Methanolobus genera were the two most abundant methanogens, with the former being predominant. Other studies suggested a key role of the methanogenic archaea
Methanosarcina in AOM via reverse methanogenesis, as in Luo et al., 2017 where the electron acceptor was Br (V) in the form of BrO
3− [88][103]. A possible explanation is the close phylogenetic relationship between ANME-2a/b, ANME-2c, ANME-2d, and ANME-3 and
Methanosarcinales [89][104]. The archaea family ANME-2d, also knowns as
Methanoperedenaceae lineage, was found to be able to oxidise methane through Mn (V) reduction in a bioreactor supplied with CH
4 and pulse of birnessite operating for 480 days
[90][105]. The inoculum originated from a bioreactor inoculated with freshwater sediment and performing AOM via Fe (III) reduction. The average Mn (II) production rate was 0.185 mmol L
−1 day
−1 with a methane consumption rate of 0.045 mmol L
−1 day
−1.