Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ana Marta Gonçalves.
Polyphenols are beneficial natural compounds with antioxidant properties that have recently gain a lot of interest for their potential therapeutic applications. Marine polyphenols derived from marine macroalgae have been discovered to possess interesting antioxidant properties; therefore, these compounds can be included in several areas of drug development.
- marine polyphenols
- seaweeds
- antioxidant activity
- neurodegenerative diseases
1. Introduction
The advantages of marine macroalgae (or seaweeds) to human wellbeing are well known [1,2,3,4][1][2][3][4]. Numerous bioactive molecules found in seaweeds may have health advantages against a range of diseases and conditions, including cancer, inflammation, microbes, and viruses [5,6,7,8,9,10,11,12][5][6][7][8][9][10][11][12]. The potential of seaweed bioactive compounds to act as a natural resource with remarkable neuroprotective properties can be based on abundant published results from recent clinical and preclinical studies. Numerous studies have documented seaweed bioactive compounds exhibiting therapeutic activities [13,14,15,16,17,18,19][13][14][15][16][17][18][19].
Seaweed biomass is a promising, renewable, and cost-effective [20,21,22][20][21][22] resource of high-value bioactive compounds that have been highly invested in within the food, pharmaceutical, and cosmetic industries [23,24,25,26,27,28,29][23][24][25][26][27][28][29].
Marine polyphenols have been discovered to be powerful antioxidant compounds; therefore, they can play a crucial role in the development of natural and innovative neuroprotective drugs.
Neuroprotection refers to methods and mechanisms that protect neuronal cells against injury, dysfunction, deterioration, and cell death in the central nervous system (CNS) [30]. These compounds may slow the progression and limit neuronal cell loss; therefore, the use of those would improve quality of life for patients affected with neurodegenerative diseases.
The progressive loss of specifically vulnerable groups of neurons characterizes neurodegenerative disorders, which are frequently (though not always) accompanied by neurodegenerative symptoms. Neurodegenerative diseases can be categorized according to their primary clinical characteristics, such as dementia, parkinsonism, or motor neuron disease; anatomical distribution of the disease, such as frontotemporal degenerations, extrapyramidal disorders, or spinocerebellar degenerations; or principal molecular abnormality [31]. Although the exact pathophysiology of neurodegenerative diseases is still unclear, common factors contribute to the disease progression: increased oxidative stress, neuroinflammation, misfolded proteins, dysfunctional mitochondria, and impaired proteostasis [32].
2. Marine Polyphenols Involved in Neuroprotective Activity
2.1. Seaweed Polyphenols
Polyphenolic secondary metabolites comprise a large collection of chemical compounds found in terrestrial plants [58,59][33][34] and seaweeds [60,61][35][36]. Tannins, a prevalent group of phenolic metabolites, contain numerous hydroxyl groups and can be classified into three groups. Condensed tannins, which are based on flavonoids, are found predominantly in woody plants, as well as in red wine and tea [62][37]. Hydrolysable tannins, formed by polyhydric alcohol, where hydroxyl groups are partly or etherified with gallic acid or related compounds, are found in some green algae and are broadly distributed in angiosperms [63][38]. Phlorotannins, one of several algal polyphenol’s groups, are of great pharmacological significance. They are composed of many phloroglucinol (1,3,5-trihydroxybenzene) (Figure 1) molecules that are linked together in various ways (Figure 2) [64][39].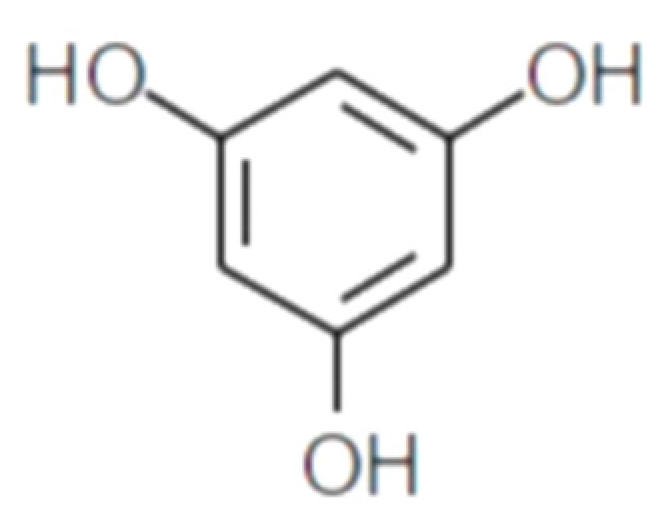
Figure 1.
Phloroglucinol (1,3,5-trihydroxybenzene) structure.
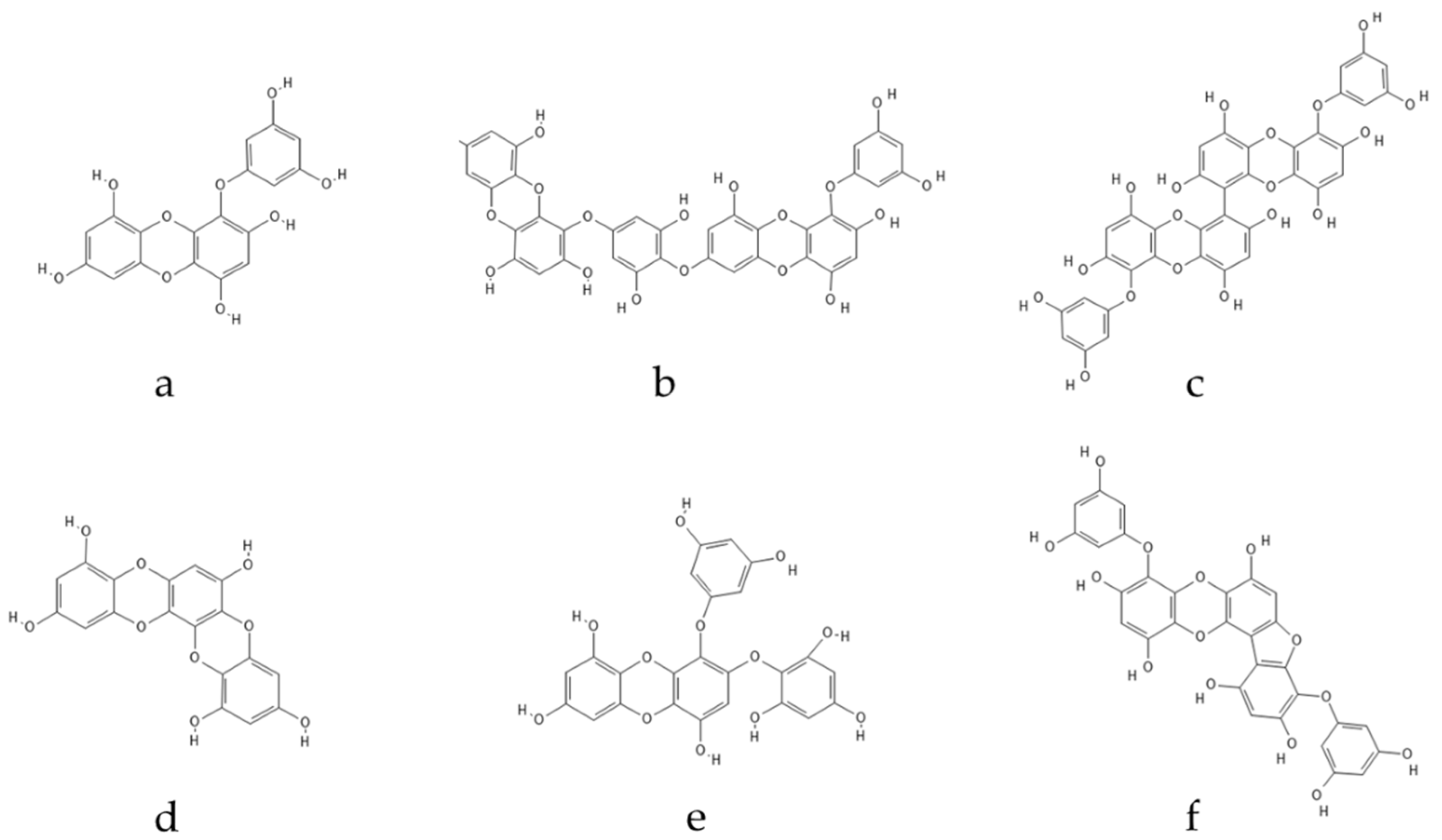
Figure 2. Eckol-class compounds: (a) eckol; (b) dieckol; (c) 6,6-bieckol; (d) dioxinodehydroeckol; (e) 2-phloroeckol; (f) phlorofucofuroeckol.
2.2. Mechanisms of Action of Antioxidant Seaweed Polyphenols
ChE inhibitors are a successful approach for treating the symptoms of neurodegenerative disorders, even though various strategies can be used to stop the progression of neurodegeneration. Phlorotannins from Ecklonia maxima were isolated by Kannan et al. [81][56], and the results showed that they had AChE inhibitory action. Dibenzo 1,4-dioxine-2,4,7,9-tetraol and eckol were found to be more effective AChE inhibitors than phloroglucinol. This is likely because they have larger molecules and more hydroxyl groups than phloroglucinol, which can modulate their interactions with AChE and subsequently block it (Figure 3). These findings highlight the potential uses of E. maxima as a beneficial ingredient that could be used as additives to foods to act as neuroprotective foods [81][56].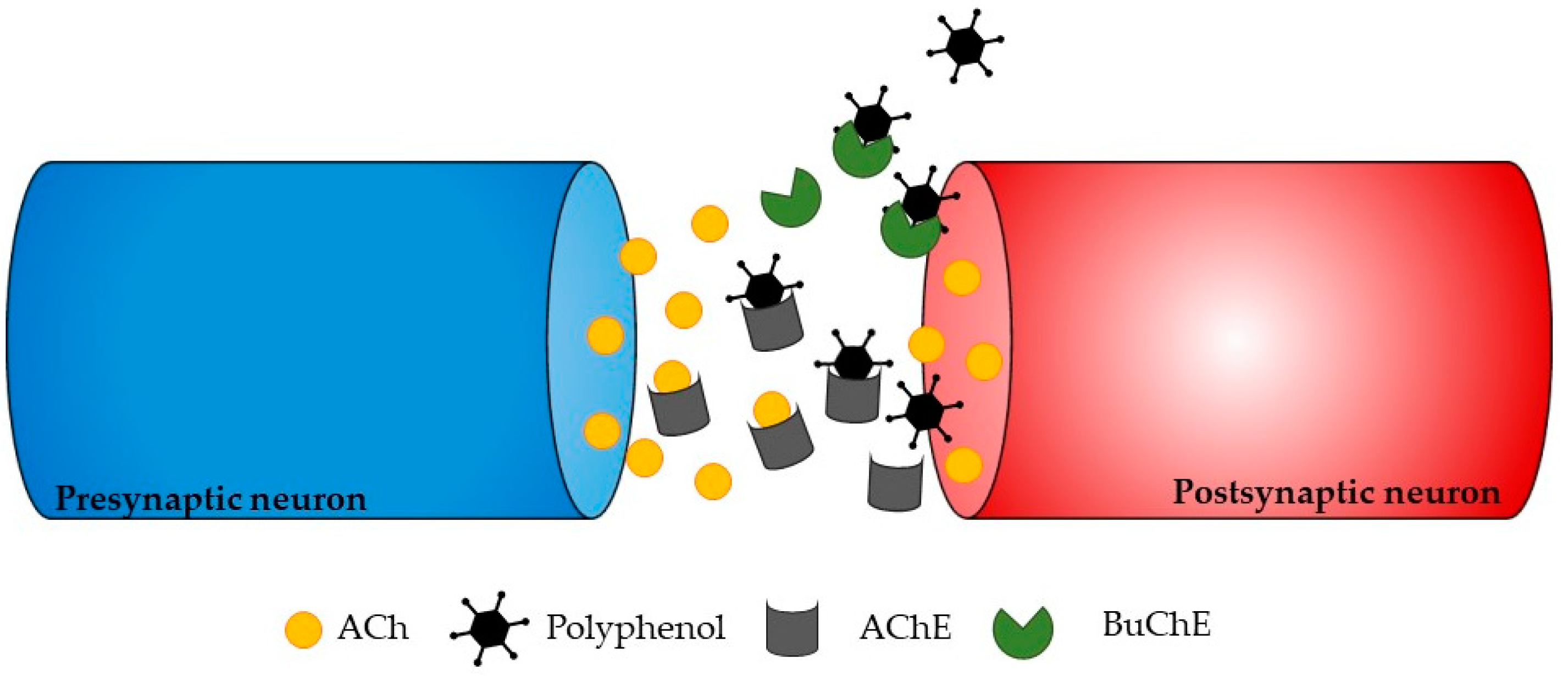
Figure 3. Illustration showing a potential way in which polyphenols may affect neurotransmission. The process of ACh formation occurs briefly before it is broken down by AChE, ultimately leading to the transmission of neurotransmitters to postsynaptic neurons. The inhibition of these enzymes occurs when polyphenols bind to the active sites of AChE or BChE.
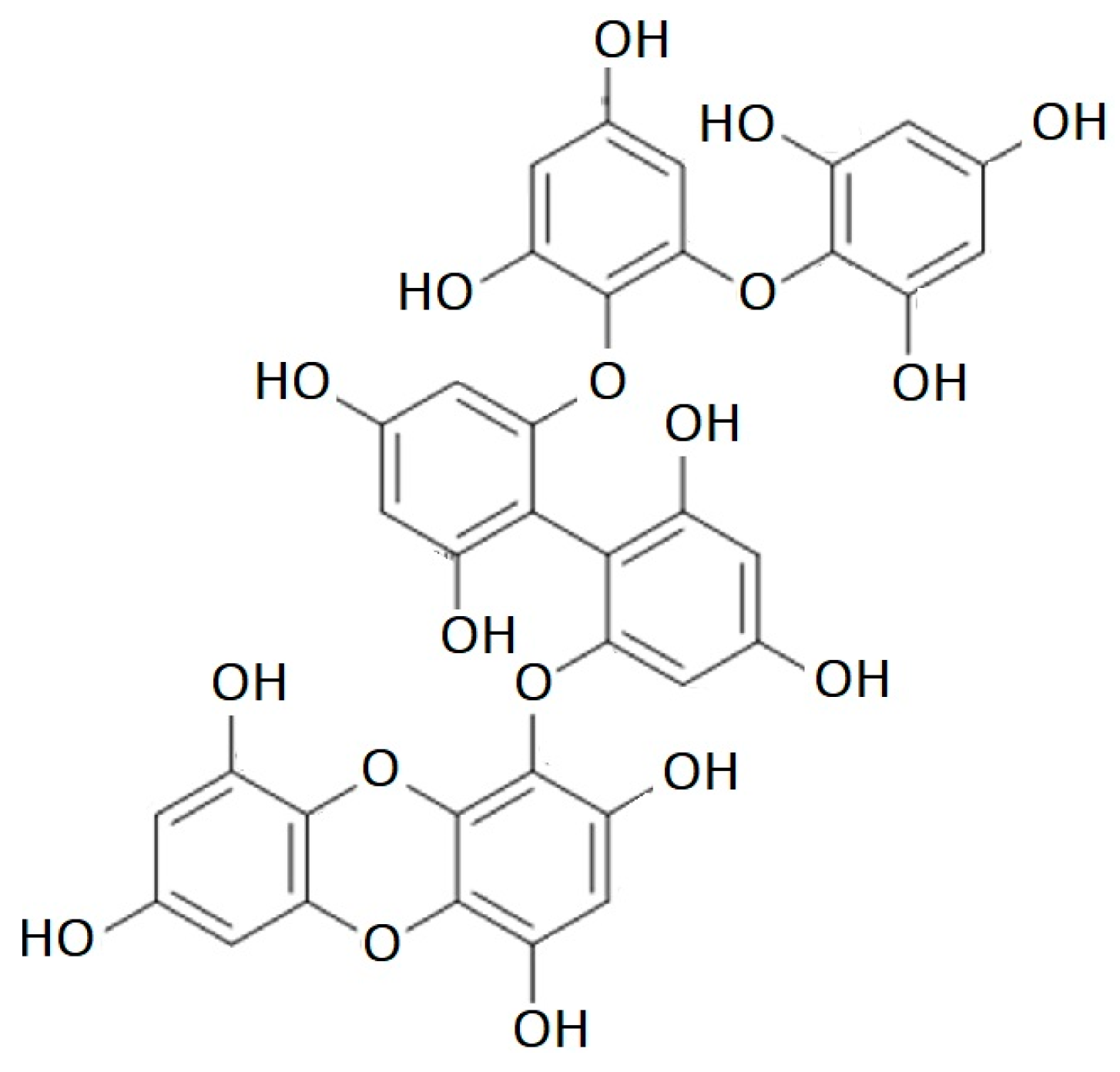
Figure 4.
Dibenzodioxin-fucodiphloroethol structure.
References
- Dhargalkar, V. Uses of Seaweeds in the Indian Diet for Sustenance and Well-Being. Sci. Cult. 2015, 80, 192–202.
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624.
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341.
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as Promising Resource of Bioactive Compounds: Overview of Novel Extraction Strategies and Design of Tailored Meat Products. Trends Food Sci. Technol. 2020, 100, 1–18.
- Cabral, E.M.; Oliveira, M.; Mondala, J.R.M.; Curtin, J.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobials from Seaweeds for Food Applications. Mar. Drugs 2021, 19, 211.
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.P.; Nishigaki, I. Cancer Preventive Efficacy of Marine Carotenoid Fucoxanthin: Cell Cycle Arrest and Apoptosis. Nutrients 2013, 5, 4978–4989.
- Sakthivel, R.; Devi, K.P. Antioxidant, Anti-Inflammatory and Anticancer Potential of Natural Bioactive Compounds from Seaweeds, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 63, ISBN 9780128179017.
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358.
- Meinita, M.D.N.; Harwanto, D.; Choi, J.S. Seaweed Exhibits Therapeutic Properties against Chronic Diseases: An Overview. Appl. Sci. 2022, 12, 2638.
- de Souza Barros, C.; Teixeira, V.L.; Paixão, I.C.N.P. Seaweeds with Anti-Herpes simplex Virus Type 1 Activity. J. Appl. Phycol. 2015, 27, 1623–1637.
- Wei, Q.; Fu, G.; Wang, K.; Yang, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Advances in Research on Antiviral Activities of Sulfated Polysaccharides from Seaweeds. Pharmaceuticals 2022, 15, 581.
- Monteiro, P.; Lomartire, S.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Call the Eckols: Present and Future Potential Cancer Therapies. Mar. Drugs 2022, 20, 387.
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential Bioactives from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491.
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222.
- Samaddar, S.; Koneri, R. Polyphenols of Marine Red Macroalga Symphyocladia latiuscula Ameliorate Diabetic Peripheral Neuropathy in Experimental Animals. Heliyon 2019, 5, e01781.
- Murphy, C.; Hotchkiss, S.; Worthington, J.; Mckeown, S.R. The Potential of Seaweed as a Source of Drugs for Use in Cancer Chemotherapy. J. Appl. Phycol. 2014, 26, 2211–2264.
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273.
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; et al. In vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. S. Afr. J. Bot. 2020, 128, 152–160.
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate Composition, Phenolic Content and in Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil under Accelerated Storage Conditions. Food Res. Int. 2017, 99, 986–994.
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528.
- Lüning, K.; Pang, S. Mass Cultivation of Seaweeds: Current Aspects and Approaches. J. Appl. Phycol. 2003, 15, 115–119.
- Emblemsvåg, J.; Kvadsheim, N.P.; Halfdanarson, J.; Koesling, M.; Nystrand, B.T.; Sunde, J.; Rebours, C. Strategic Considerations for Establishing a Large-Scale Seaweed Industry Based on Fish Feed Application: A Norwegian Case Study. J. Appl. Phycol. 2020, 32, 4159–4169.
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8.
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688.
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.S.; Jena, M. Beneficial Effects of Seaweeds and Seaweed-Derived Bioactive Compounds: Current Evidence and Future Prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242.
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated Polysaccharides and Its Commercial Applications in Food Industries—A Review. J. Food Sci. Technol. 2021, 58, 2453–2466.
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140.
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331.
- Pereira, L.; Critchley, A.T. The COVID 19 Novel Coronavirus Pandemic 2020: Seaweeds to the Rescue? Why Does Substantial, Supporting Research about the Antiviral Properties of Seaweed Polysaccharides Seem to Go Unrecognized by the Pharmaceutical Community in These Desperate Times? J. Appl. Phycol. 2020, 32, 1875–1877.
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, Oxidative Stress and Mitochondrial Dysfunction: Cross Talk in Alzheimer’s Disease and Parkinson’s Disease. Drug Des. Dev. Ther. 2017, 11, 797–810.
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035.
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116.
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128135723.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278.
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins Are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273.
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochem. 2011, 46, 2219–2224.
- Kılıç, C.; Can, Z.; Yılmaz, A.; Yıldız, S.; Turna, H. Antioxidant Properties of Some Herbal Teas (Green Tea, Senna, Corn Silk, Rosemary) Brewed at Different Temperatures. Int. J. Second. Metab. 2017, 4, 148–154.
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and Tannin-like Compounds-Nature, Occurrence, Dietary Intake and Effects on Nutrition and Health. J. Sci. Food Agric. 2000, 80, 1094–1117.
- Thomas, N.V.; Kim, S.K. Potential Pharmacological Applications of Polyphenolic Derivatives from Marine Brown Algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335.
- Lopes, G.L.L. Seaweeds from the Portuguese Coast: Chemistry, Antimicrobial and Anti-Inflammatory Capacity. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2014; pp. 1–24.
- Arnold, T.M.; Targett, N.M. Marine Tannins: The Importance of a Mechanistic Framework for Predicting Ecological Roles. J. Chem. Ecol. 2002, 28, 1919–1934.
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145.
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant Properties of Phlorotannins from Brown Seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614.
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The Anti-Inflammatory Effects of Phlorotannins from Eisenia arborea on Mouse Ear Edema by Inflammatory Inducers. J. Funct. Foods 2013, 5, 2019–2023.
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Yamada, Y.; Imai, K.; Ito, T.; Kakinuma, M.; Amano, H. The Inhibitory Effects of Components from a Brown Alga, Eisenia arborea, on Degranulation of Mast Cells and Eicosanoid Synthesis. J. Funct. Foods 2009, 1, 387–393.
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial Effect of Phlorotannins from Marine Brown Algae. Food Chem. Toxicol. 2012, 50, 3251–3255.
- Lee, S.H.; Jeon, Y.J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136.
- Lee, J.Y.; Kim, S.M.; Jung, W.S.; Song, D.G.; Um, B.H.; Son, J.K.; Pan, C.H. Phlorofucofuroeckol-A, a Potent Inhibitor of Aldo-Keto Reductase Family 1 Member B10, from the Edible Brown Alga Eisenia bicyclis. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 721–727.
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Mason, P.K. Phlorotannin-Protein Interactions. J. Chem. Ecol. 1996, 22, 1877–1899.
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-Based Natural Ingredients: Stability of Phlorotannins during Extraction, Storage, Passage through the Gastrointestinal Tract and Potential Incorporation into Functional Foods. Food Res. Int. 2020, 137, 109676.
- Heo, S.; Park, E.; Lee, K.; Jeon, Y. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623.
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol Isolated from Ecklonia cava Attenuates Oxidative Stress Induced Cell Damage in Lung Fibroblast Cells. FEBS Lett. 2005, 579, 6295–6304.
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava Protects V79-4 Lung Fibroblast against Hydrogen Peroxide Induced Cell Damage. Free Radic. Res. 2005, 39, 883–892.
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective Effect of Phloroglucinol on Oxidative Stress Induced Cell Damage via Catalase Activation. J. Cell. Biochem. 2006, 97, 609–620.
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant Activities of Phlorotannins Isolated from Japanese Laminariaceae. In Advances in Cultivation of Gelidiales; Springer: Berlin/Heidelberg, Germany, 2007; pp. 255–261. ISBN 9781402096198.
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Van Staden, J. Acetylcholinesterase Inhibitory Activity of Phlorotannins Isolated from the Brown Alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254.
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.L.W. Inhibitory Effects and Molecular Mechanism of Dieckol Isolated from Marine Brown Alga on COX-2 and INOS in Microglial Cells. J. Agric. Food Chem. 2009, 57, 4439–4446.
- Yoon, N.Y.; Lee, S.H.; Yong-Li; Kim, S.K. Phlorotannins from Ishige okamurae and Their Acetyl- and Butyrylcholinesterase Inhibitory Effects. J. Funct. Foods 2009, 1, 331–335.
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs 2020, 18, 654.
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.R.; Yoon, E.; Kim, O.Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Drugs 2019, 17, 7.
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective Effect of Edible Brown Alga Eisenia bicyclis on Amyloid Beta Peptide-Induced Toxicity in PC12 Cells. Arch. Pharm. Res. 2012, 35, 1989–1998.
- Huber, C.M.; Yee, C.; May, T.; Dhanala, A.; Mitchell, C.S. Cognitive Decline in Preclinical Alzheimer’s Disease: Amyloid-Beta versus Tauopathy. J. Alzheimer’s Dis. 2018, 61, 265–281.
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2.
- Sun, X.; Chen, W.D.; Wang, Y.D. β-Amyloid: The Key Peptide in the Pathogenesis of Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 221.
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava-an in vitro and in silico Study. Mar. Drugs 2019, 17, 91.
- Shrestha, S.; Johnston, M.R.; Zhang, W.; Smid, S.D. A Phlorotannin Isolated from Ecklonia radiata, Dibenzodioxin-Fucodiphloroethol, Inhibits Neurotoxicity and Aggregation of β-Amyloid. Phytomedicine Plus 2021, 1, 100125.
More
