Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ana Marta Gonçalves and Version 2 by Camila Xu.
Polyphenols are beneficial natural compounds with antioxidant properties that have recently gain a lot of interest for their potential therapeutic applications. Marine polyphenols derived from marine macroalgae have been discovered to possess interesting antioxidant properties; therefore, these compounds can be included in several areas of drug development.
- marine polyphenols
- seaweeds
- antioxidant activity
- neurodegenerative diseases
1. Introduction
The advantages of marine macroalgae (or seaweeds) to human wellbeing are well known [1][2][3][4][1,2,3,4]. Numerous bioactive molecules found in seaweeds may have health advantages against a range of diseases and conditions, including cancer, inflammation, microbes, and viruses [5][6][7][8][9][10][11][12][5,6,7,8,9,10,11,12]. The potential of seaweed bioactive compounds to act as a natural resource with remarkable neuroprotective properties can be based on abundant published results from recent clinical and preclinical studies. Numerous studies have documented seaweed bioactive compounds exhibiting therapeutic activities [13][14][15][16][17][18][19][13,14,15,16,17,18,19].
Seaweed biomass is a promising, renewable, and cost-effective [20][21][22][20,21,22] resource of high-value bioactive compounds that have been highly invested in within the food, pharmaceutical, and cosmetic industries [23][24][25][26][27][28][29][23,24,25,26,27,28,29].
Marine polyphenols have been discovered to be powerful antioxidant compounds; therefore, they can play a crucial role in the development of natural and innovative neuroprotective drugs.
Neuroprotection refers to methods and mechanisms that protect neuronal cells against injury, dysfunction, deterioration, and cell death in the central nervous system (CNS) [30]. These compounds may slow the progression and limit neuronal cell loss; therefore, the use of those would improve quality of life for patients affected with neurodegenerative diseases.
The progressive loss of specifically vulnerable groups of neurons characterizes neurodegenerative disorders, which are frequently (though not always) accompanied by neurodegenerative symptoms. Neurodegenerative diseases can be categorized according to their primary clinical characteristics, such as dementia, parkinsonism, or motor neuron disease; anatomical distribution of the disease, such as frontotemporal degenerations, extrapyramidal disorders, or spinocerebellar degenerations; or principal molecular abnormality [31]. Although the exact pathophysiology of neurodegenerative diseases is still unclear, common factors contribute to the disease progression: increased oxidative stress, neuroinflammation, misfolded proteins, dysfunctional mitochondria, and impaired proteostasis [32].
2. Marine Polyphenols Involved in Neuroprotective Activity
2.1. Seaweed Polyphenols
Polyphenolic secondary metabolites comprise a large collection of chemical compounds found in terrestrial plants [33][34][58,59] and seaweeds [35][36][60,61]. Tannins, a prevalent group of phenolic metabolites, contain numerous hydroxyl groups and can be classified into three groups. Condensed tannins, which are based on flavonoids, are found predominantly in woody plants, as well as in red wine and tea [37][62]. Hydrolysable tannins, formed by polyhydric alcohol, where hydroxyl groups are partly or etherified with gallic acid or related compounds, are found in some green algae and are broadly distributed in angiosperms [38][63]. Phlorotannins, one of several algal polyphenol’s groups, are of great pharmacological significance. They are composed of many phloroglucinol (1,3,5-trihydroxybenzene) (Figure 1) molecules that are linked together in various ways (Figure 2) [39][64].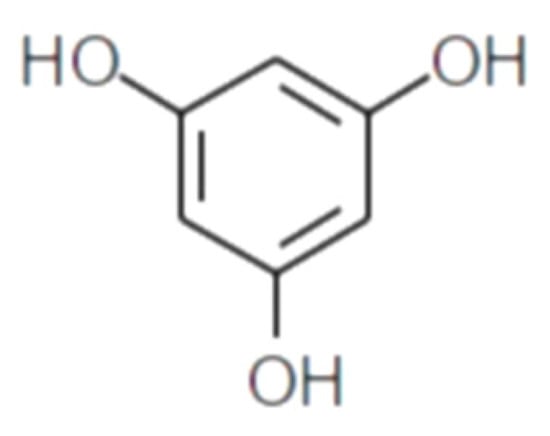
Figure 1.
Phloroglucinol (1,3,5-trihydroxybenzene) structure.
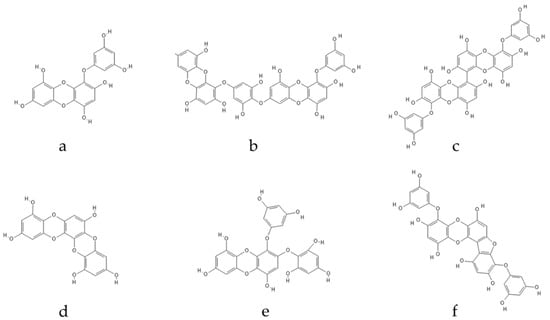
Figure 2. Eckol-class compounds: (a) eckol; (b) dieckol; (c) 6,6-bieckol; (d) dioxinodehydroeckol; (e) 2-phloroeckol; (f) phlorofucofuroeckol.
2.2. Mechanisms of Action of Antioxidant Seaweed Polyphenols
ChE inhibitors are a successful approach for treating the symptoms of neurodegenerative disorders, even though various strategies can be used to stop the progression of neurodegeneration. Phlorotannins from Ecklonia maxima were isolated by Kannan et al. [56][81], and the results showed that they had AChE inhibitory action. Dibenzo 1,4-dioxine-2,4,7,9-tetraol and eckol were found to be more effective AChE inhibitors than phloroglucinol. This is likely because they have larger molecules and more hydroxyl groups than phloroglucinol, which can modulate their interactions with AChE and subsequently block it (Figure 3). These findings highlight the potential uses of E. maxima as a beneficial ingredient that could be used as additives to foods to act as neuroprotective foods [56][81].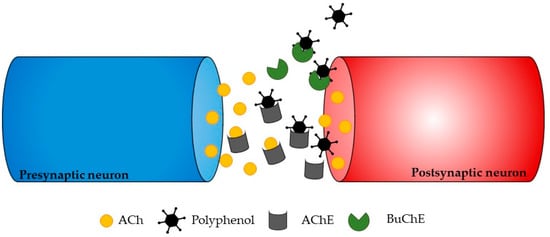
Figure 3. Illustration showing a potential way in which polyphenols may affect neurotransmission. The process of ACh formation occurs briefly before it is broken down by AChE, ultimately leading to the transmission of neurotransmitters to postsynaptic neurons. The inhibition of these enzymes occurs when polyphenols bind to the active sites of AChE or BChE.
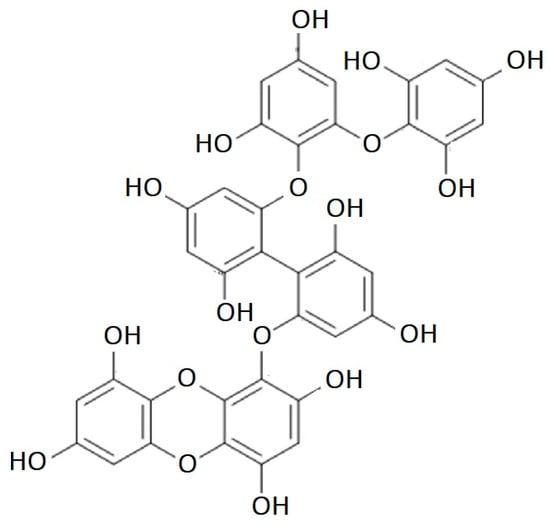
Figure 4.
Dibenzodioxin-fucodiphloroethol structure.
