Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Jialong Shen.
Membranes are a promising technology platform for CO2 capture because they are modular, scalable, and compact. This makes them desirable for process intensification and reducing energy costs. Biocatalytic membranes encompass many different types of materials and functionality.
- carbonic anhydrase
- CO2 capture
- CO2 reduction
- enzyme
- membranes
- formate dehydrogenase
1. Introduction
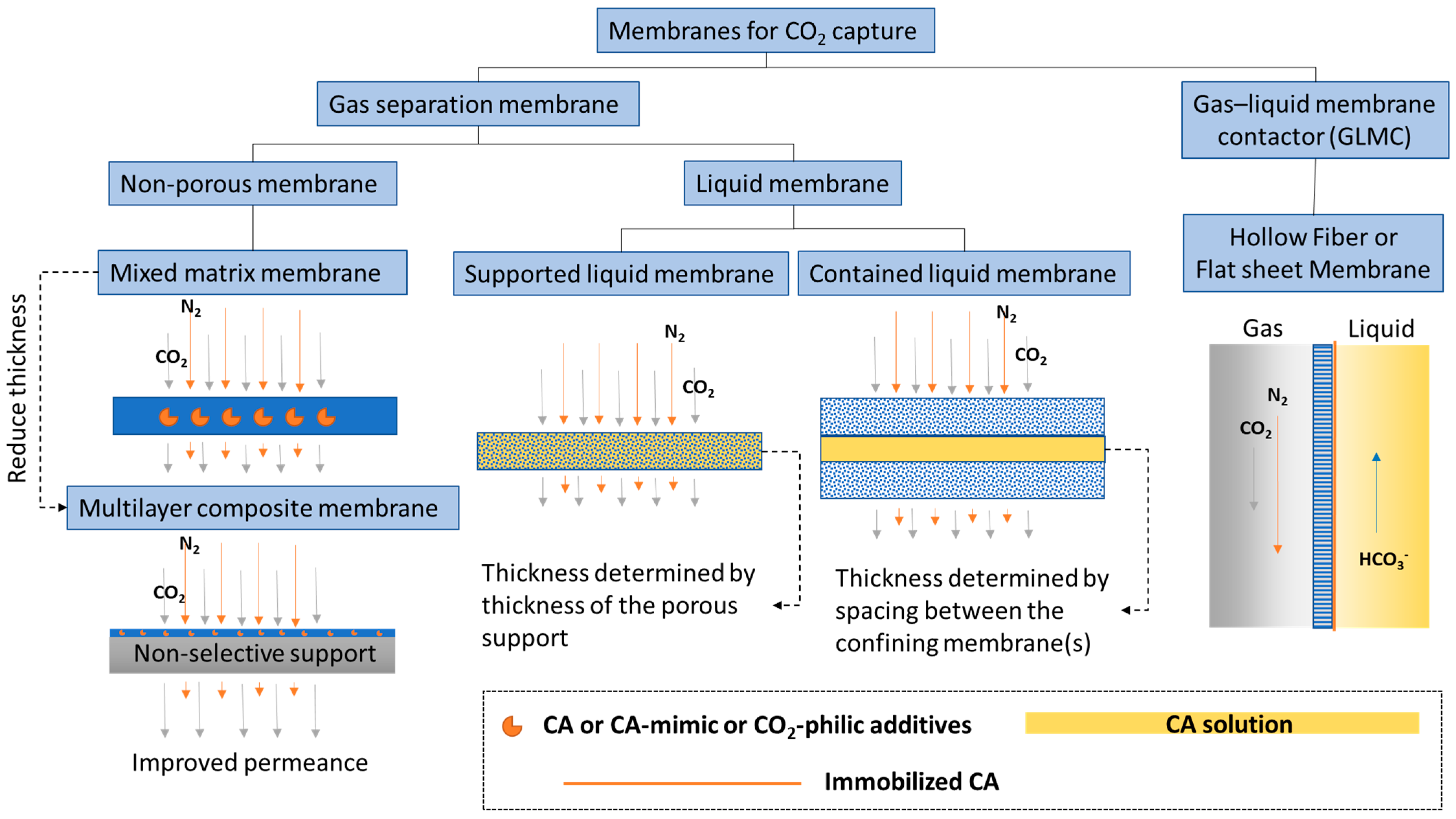
Membranes are a promising technology platform for CO2 capture because they are modular, scalable, and compact. This makes them desirable for process intensification and reducing energy costs [54,55][1][2]. Membranes encompass many different types of materials and functionality. Here, to distinguish biocatalytic membranes according to their configurations and separation mechanisms, membranes are loosely divided into two categories, based on the physical states of the fluids separated by the membrane: CO2 gas separation membranes and CO2 gas–liquid membrane contactors (Figure 1).
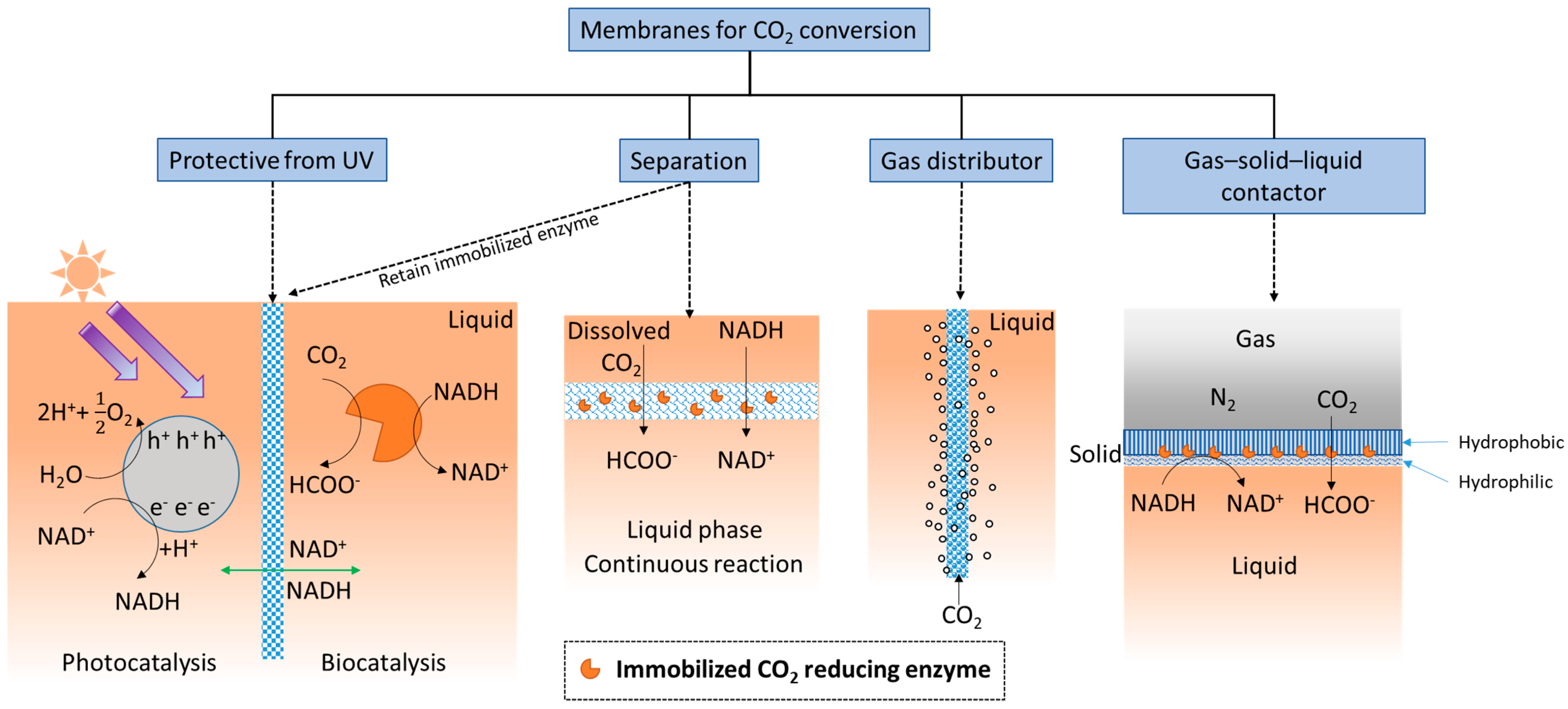 Additionally, membranes provide ample surface area for enzymes to be immobilized, and therefore, can provide high catalytic enhancement. Considering the membrane’s separation function, when substrate is delivered as dissolved CO2-saturated water [35[41][42],36], the membrane structure creates a localized environment where the CO2 conversion reaction can take place continuously in the liquid phase. Membranes can separate either dissolved or immobilized biocatalysts from products [38,39][43][44] (exemplified by two schematics under “Separation” in Figure 2). The importance of this seemingly simple function of solid–liquid separation and recovery of enzymes should not be underestimated. An evaluation of using ultrafiltration membranes to separate dissolved enzymes from a CO2-rich solvent [93][45], to avoid pumping the enzymes into a high temperature desorber for solvent regeneration, found that even with an enzyme retention rate as high as 99.9%, only 50% of the enzymes are retained after 1 month of operation. Therefore, strategies that prevent enzymes from leaching through or away from membranes can be critical. Biocatalyst retention by immobilization is especially important for operating enzymatic membrane reactors for CO2 reduction catalyzed by oxidoreductases.
As illustrated under ‘gas distributor’ in Figure 2, porous membranes, specifically porous hollow fiber membranes, can be used to infuse gaseous CO2 into the reaction medium [94][46] to increase the availability of soluble CO2. This approach is often used in conjunction with adjacent sets of hollow fiber membranes with immobilized enzymes attached [95][47]. In addition, since gaseous CO2 is attracted to hydrophobic surfaces, amphiphilic membranes functioning as gas–solid–liquid contactors (Figure 2, right schematic) have recently been developed for converting gaseous CO2 into water soluble formic acid [96][48].
Additionally, membranes provide ample surface area for enzymes to be immobilized, and therefore, can provide high catalytic enhancement. Considering the membrane’s separation function, when substrate is delivered as dissolved CO2-saturated water [35[41][42],36], the membrane structure creates a localized environment where the CO2 conversion reaction can take place continuously in the liquid phase. Membranes can separate either dissolved or immobilized biocatalysts from products [38,39][43][44] (exemplified by two schematics under “Separation” in Figure 2). The importance of this seemingly simple function of solid–liquid separation and recovery of enzymes should not be underestimated. An evaluation of using ultrafiltration membranes to separate dissolved enzymes from a CO2-rich solvent [93][45], to avoid pumping the enzymes into a high temperature desorber for solvent regeneration, found that even with an enzyme retention rate as high as 99.9%, only 50% of the enzymes are retained after 1 month of operation. Therefore, strategies that prevent enzymes from leaching through or away from membranes can be critical. Biocatalyst retention by immobilization is especially important for operating enzymatic membrane reactors for CO2 reduction catalyzed by oxidoreductases.
As illustrated under ‘gas distributor’ in Figure 2, porous membranes, specifically porous hollow fiber membranes, can be used to infuse gaseous CO2 into the reaction medium [94][46] to increase the availability of soluble CO2. This approach is often used in conjunction with adjacent sets of hollow fiber membranes with immobilized enzymes attached [95][47]. In addition, since gaseous CO2 is attracted to hydrophobic surfaces, amphiphilic membranes functioning as gas–solid–liquid contactors (Figure 2, right schematic) have recently been developed for converting gaseous CO2 into water soluble formic acid [96][48].

Figure 1. Categories of biocatalytic membranes used for CO2 capture.
2. CO2 Separation Membrane
A membrane that separates two gas phases on either side—CO2 lean gas mixture on the feed and CO2 enriched gas phase on the permeate side—is called a CO2 separation membrane. This category encompasses a large selection of membrane types from non-porous glassy polymer membranes, fixed-site carrier membranes [56][3], and ultrathin nanocomposite membranes [57][4], to contained liquid membranes [58][5]. Research efforts on CO2 separation membranes have focused on improving performance-limiting membrane properties, such as CO2 gas permeance and selectivity [59][6]. New classes of polymer materials, such as polymers of intrinsic microporosity (PIM) [60,61][7][8] and ladder polymers [62][9], have been invented that show superior CO2 separation properties well above the empirical Robeson upper-bound [63][10], which classically delineates the trade-off relationship between gas permeability and selectivity. However, physical aging is still an issue that needs to be solved. This problem is common to all glassy polymer membranes, including in the new classes of materials, albeit to a lesser extent owing to the presence of inherent structural porosities. In one case, treatment with super critical CO2 altered the internal structure of a PIM, leading to decreased CO2 permeance [64][11]. In another case, after being physically aged, ladder polymers showed increased selectivity but decreased permeability [62][9], indicating a decreased free volume. To alleviate physical aging issues in glassy non-porous polymer membranes, inorganic aging-resistant CO2-philic components are added to the polymer matrix to form mixed matrix membranes (MMM). Recently, Tan et al. [65][12] discovered a new method for adding high loadings of zeolite into a polyimide membrane matrix that achieved a CO2/CH4 mixed-gas selectivity of ~423 and a CO2 permeability of ~8300 Barrer at moderate pressure and ambient temperature. To put these numbers in perspective, at a similar CO2/CH4 selectivity of 400, the 2008 Robeson upper-bound for the CO2/CH4 pair anticipates a CO2 permeability of only ~1 Barrer [63][10]. In order to improve the overall sustainability profile of CO2 separation technologies, biopolymer-based MMM, such as chitosan-based non-porous membranes, have recently emerged as alternatives to conventional non-renewable polymer matrices [66][13]. Casado-Coterillo et al. [67][14] fabricated a chitosan MMM filled with metal organic framework (MOF) and non-toxic ionic liquid that achieved a high permeability of 4754–5413 Barrer (or 47–52 GPU) and a CO2/N2 selectivity of 12–19. Borgohain et al. [68][15] synthesized carboxymethyl chitosan as a matrix for compatibilization with scarcely soluble multi-walled carbon nanotubes (MWCNT) to make a thin MMM selective layer (2.7 µm) that exhibited a CO2 permeance of 43 GPU and a CO2/N2 selectivity of 45. The hydrophilicity and free amine groups of the chitosan material could be contributing to the excellent CO2 transport properties, especially in humidified conditions, compared with the commercial hydrophobic membranes [69][16]. Owing to their abundance in nature, tailorable functional groups, and excellent membrane forming properties, chitosan [70][17] and other polysaccharides [71[18][19],72], could play an increasing role in the fabrication of novel CO2 separation membranes. Another way to improve membrane performance is by making thin film composites (TFC) [73][20] or integrated multilayer membranes [74][21], both with ultra-thin CO2 selective layers for facilitated CO2 transport. CA and CA mimics have been successfully used to construct both MMM and thin CO2 selective layers for facilitated CO2 separation [75,76][22][23]. However, these advanced facilitated transport membranes are still at lab-scale and no direct comparison between these and commercial scale CO2 chemical absorption processes is available in the literature. Nevertheless, a recent techno-economic analysis (TEA) study compared a non-facilitated polymeric membrane process (Membrane Technology and Research, Inc., Newark, CA, USA) [77][24] to an enzyme-based chemical absorption process (Akermin Inc., St. Louis, MO, USA) and found that the latter is economically more attractive in a simulated CO2 capture scenario from a 600 MWe power plant flue gas. This result emphasizes the potential for enzymes to improve energy efficiency of conventional energy intensive processes. Interestingly, the study also predicted that the membrane technology could become more efficient if CO2 permeance at low pressure (<1.5 bar) could be enhanced. Because CA is already particularly efficient at converting CO2 to bicarbonate at ambient pressure, developing low pressure facilitated CO2 transport membranes that utilize the fast enzyme reaction rate is a promising concept. Liquid membranes that separate two gas phases are also defined as CO2 separation membranes. CA plays a similar CO2 hydration facilitator role in liquid membranes, provided there is water present. General types of liquid membranes include supported liquid membranes and contained liquid membranes (Figure 1). Sometimes distinctions are made between supported liquid membranes (SLM) and immobilized liquid membranes (ILM), where in the first case, liquid fills spaces between fibers in the membrane and the second case, liquid fills specific pores in the membrane [78][25]. However, most of the time, these two nomenclatures are used interchangeably. Disadvantages of common SLMs or ILMs include the formation of gravity-induced downward bulges in the liquid phase (called catenary curves), low tolerance to transmembrane pressure differences, and a high evaporation tendency. All of these problems can be alleviated by contained liquid membrane configurations in which liquid is bound by porous membrane surfaces [78][25]. Different types of liquid can be used to construct liquid membranes, including hydrogels [79][26], ionic liquids [80][27], deep eutectic solvents [81][28], and aqueous buffers [82][29]. Both flat sheet and hollow fiber membranes are commonly used.3. CO2 Liquid Contactor Membrane
A membrane that separates a gas phase containing CO2 from a liquid phase where CO2 is absorbed, is categorized as a CO2 liquid contactor membrane (Figure 1). This category emerged as a new hybrid membrane system, called gas–liquid membrane contactors (GLMC), that combines the modularity and high surface area of the membrane with the high selectivity of the chemical absorption process [83,84][30][31]. Non-enzymatic GLMC developments have focused on improving membrane stability [85][32], minimizing pore wetting [86][33], and selecting the best solvent and activator [9][34]. Reviews of modeling methods used to analyze the mass transfer in hollow fiber gas–liquid membrane contactors (HFGLMC) for post-combustion carbon capture are available [83][30]. Improvements to membrane materials were also explored by blending polysulfone (PSf) with PEI, a CO2-philic polymer. The observed optimal additive ratio for higher capture performance was attributed to chemical affinity, whereas non-optimal conditions inadvertently caused pore wetting and clogging by K2CO3 precipitation [87][35]. Another way to improve GLMC performance is increasing the mass transfer of CO2 at the gas–liquid interface catalyzed by CA enzymes, which are either immobilized on the membrane [88][36], dissolved in the solvent [89][37], or immobilized both on the membrane and on mobile nanoparticles dispersed in the solvent for additional process intensification [90][38]. A recent TEA study compared a CA-immobilized hollow fiber membrane contactor (HFMC) with benign solvent and vacuum-assisted solvent regeneration with the benchmark case where monoethanolamine (MEA) was used in a conventional packed column process. The projection estimated that at 90% CO2 capture from a 685 MWe coal-fired power plant, the enzymatic process achieved a 43% reduction in energy consumption of the capture and compression unit, a 31% reduction in capital cost (CAPEX), and a 28% reduction in operating expenses (OPEX) in comparison with the MEA benchmark [91][39].4. Other Membrane Structure Functions
The simplest definition of a membrane is a thin layer that acts as a boundary or barrier. This barrier can prevent random mass exchange based on size or physical phase, or can provide protection against harsh environments. Membranes used for CO2 conversion and utilization applications may require different or added functionality compared with those used for CO2 capture. For example, as shown in Figure 2, an ultraviolet (UV) protective membrane was used to block UV irradiation and simultaneously retain enzymes (based on their large size) on the biocatalysis side [92][40], while allowing small cofactor molecules to freely pass between the separate photocatalytic and biocatalytic reaction chambers.
Figure 2. Functionalities of membranes used for biocatalytic CO2 conversion.
References
- He, X. A Review of Material Development in the Field of Carbon Capture and the Application of Membrane-Based Processes in Power Plants and Energy-Intensive Industries. Energy. Sustain. Soc. 2018, 8, 34.
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663.
- He, X.; Lindbråthen, A.; Kim, T.J.; Hägg, M.B. Pilot Testing on Fixed-Site-Carrier Membranes for CO2 Capture from Flue Gas. Int. J. Greenh. Gas Control 2017, 64, 323–332.
- Merkel, T.; Kniep, J.; Wei, X.; Carlisle, T.; White, S.; Pande, S.; Fulton, D.; Watson, R.; Hoffman, T.; Freeman, B.; et al. Pilot Testing of a Membrane System for Postcombustion CO2 Capture; National Energy Technology Laboratory: Pittsburgh, PA, USA; Morgantown, WV, USA, 2015.
- Trachtenberg, M.C.; Cowan, R.M.; Smith, D.A.; Horazak, D.A.; Jensen, M.D.; Laumb, J.D.; Vucelic, A.P.; Chen, H.; Wang, L.; Wu, X. Membrane-Based, Enzyme-Facilitated, Efficient Carbon Dioxide Capture. Energy Procedia 2009, 1, 353–360.
- Zhou, F.; Tien, H.N.; Xu, W.L.; Chen, J.T.; Liu, Q.; Hicks, E.; Fathizadeh, M.; Li, S.; Yu, M. Ultrathin Graphene Oxide-Based Hollow Fiber Membranes with Brush-like CO2-Philic Agent for Highly Efficient CO2 Capture. Nat. Commun. 2017, 8, 2107.
- McKeown, N.B. Polymers of Intrinsic Microporosity (PIMs). Polymer 2020, 202, 122736.
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson Upper Bounds for CO2/CH4 and CO2/N2 Separations Using a Series of Ultrapermeable Benzotriptycene-Based Polymers of Intrinsic Microporosity. Energy Environ. Sci. 2019, 12, 2733–2740.
- Lai, H.W.H.; Benedetti, F.M.; Ahn, J.M.; Robinson, A.M.; Wang, Y.; Pinnau, I.; Smith, Z.P.; Xia, Y. Hydrocarbon Ladder Polymers with Ultrahigh Permselectivity for Membrane Gas Separations. Science 2022, 375, 1390–1392.
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400.
- Scholes, C.A.; Kanehashi, S. Polymer of Intrinsic Microporosity (PIM-1) Membranes Treated with Supercritical CO2. Membranes 2019, 9, 41.
- Tan, X.; Robijns, S.; Thür, R.; Ke, Q.; De Witte, N.; Lamaire, A.; Li, Y.; Aslam, I.; Van Havere, D.; Donckels, T.; et al. Truly Combining the Advantages of Polymeric and Zeolite Membranes for Gas Separations. Science 2022, 378, 1189–1194.
- Torre-Celeizabal, A.; Casado-Coterillo, C.; Garea, A. Biopolymer-Based Mixed Matrix Membranes (MMMs) for CO2/CH4 Separation: Experimental and Modeling Evaluation. Membranes 2022, 12, 561.
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and Characterisation of MOF/Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Separation. RSC Adv. 2015, 5, 102350–102361.
- Borgohain, R.; Jain, N.; Prasad, B.; Mandal, B.; Su, B. Carboxymethyl Chitosan/Carbon Nanotubes Mixed Matrix Membranes for CO2 Separation. React. Funct. Polym. 2019, 143, 104331.
- Casado-Coterillo, C.; Fernández-Barquín, A.; Irabien, A. Effect of Humidity on CO2/N2 and CO2/CH4 Separation Using Novel Robust Mixed Matrix Composite Hollow Fiber Membranes: Experimental and Model Evaluation. Membranes 2019, 10, 6.
- Shen, J.; Nada, A.A.; Abou-Zeid, N.Y.; Hudson, S.M. Synthesis of Chitosan Iodoacetamides via Carbodiimide Coupling Reaction: Effect of Degree of Substitution on the Hemostatic Properties. Carbohydr. Polym. 2020, 229, 115522.
- Nada, A.A.; Ali, E.A.; Soliman, A.A.F.; Shen, J.; Abou-Zeid, N.Y.; Hudson, S.M. Multi-Layer Dressing Made of Laminated Electrospun Nanowebs and Cellulose-Based Adhesive for Comprehensive Wound Care. Int. J. Biol. Macromol. 2020, 162, 629–644.
- Nada, A.A.; Abdellatif, F.H.H.; Soliman, A.A.F.; Shen, J.; Hudson, S.M.; Abou-Zeid, N.Y. Fabrication and Bioevaluation of a Medicated Electrospun Mat Based on Azido-Cellulose Acetate via Click Chemistry. Cellulose 2019, 26, 9721–9736.
- Ji, Y.; Zhang, M.; Guan, K.; Zhao, J.; Liu, G.; Jin, W. High-Performance CO2 Capture through Polymer-Based Ultrathin Membranes. Adv. Funct. Mater. 2019, 29, 1–9.
- Sandru, M.; Sandru, E.M.; Ingram, W.F.; Deng, J.; Stenstad, P.M.; Deng, L.; Spontak, R.J. An Integrated Materials Approach to Ultrapermeable and Ultraselective CO2 Polymer Membranes. Science 2022, 376, 90–94.
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-Embedded Metal-Organic Framework Membranes on Polymeric Substrates for Efficient CO2 Capture. J. Mater. Chem. A 2017, 5, 19954–19962.
- Saeed, M.; Deng, L. CO2 Facilitated Transport Membrane Promoted by Mimic Enzyme. J. Memb. Sci. 2015, 494, 196–204.
- Gilassi, S.; Taghavi, S.M.; Rodrigue, D.; Kaliaguine, S. Techno-Economic Evaluation of Membrane and Enzymatic-Absorption Processes for CO2 Capture from Flue-Gas. Sep. Purif. Technol. 2020, 248, 116941.
- Bao, L.; Trachtenberg, M.C. Facilitated Transport of CO2 across a Liquid Membrane: Comparing Enzyme, Amine, and Alkaline. J. Memb. Sci. 2006, 280, 330–334.
- Cheng, L.H.; Zhang, L.; Chen, H.L.; Gao, C.J. Hollow Fiber Contained Hydrogel-CA Membrane Contactor for Carbon Dioxide Removal from the Enclosed Spaces. J. Memb. Sci. 2008, 324, 33–43.
- Bednár, A.; Nemestóthy, N.; Bakonyi, P.; Fülöp, L.; Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K.; Bélafi-Bakó, K. Enzymatically-Boosted Ionic Liquid Gas Separation Membranes Using Carbonic Anhydrase of Biomass Origin. Chem. Eng. J. 2016, 303, 621–626.
- De Castro, A.M.; Prasavath, D.; Bevilaqua, J.V.; Portugal, C.A.M.; Neves, L.A.; Crespo, J.G. Role of Water on Deep Eutectic Solvents (DES) Properties and Gas Transport Performance in Biocatalytic Supported DES Membranes. Sep. Purif. Technol. 2021, 255, 117763.
- Nemestóthy, N.; Bakonyi, P.; Németh, Z.; Bélafi-Bakó, K. Evaluation of Pectin-Reinforced Supported Liquid Membranes Containing Carbonic Anhydrase: The Role of Ionic Liquid on Enzyme Stability and CO2 Separation Performance. J. CO2 Util. 2018, 24, 59–63.
- Rivero, J.R.; Panagakos, G.; Lieber, A.; Hornbostel, K. Hollow Fiber Membrane Contactors for Post-Combustion Carbon Capture: A Review of Modeling Approaches. Membranes 2020, 10, 382.
- Vadillo, J.M.; Gómez-Coma, L.; Garea, A.; Irabien, A. Hollow Fiber Membrane Contactors in CO2 Desorption: A Review. Energy Fuels 2021, 35, 111–136.
- Porcheron, F.; Ferré, D.; Favre, E.; Nguyen, P.T.; Lorain, O.; Mercier, R.; Rougeau, L. Hollow Fiber Membrane Contactors for CO2 Capture: From Lab-Scale Screening to Pilot-Plant Module Conception. Energy Procedia 2011, 4, 763–770.
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van Der Bruggen, B. CO2 Capture Using Hollow Fiber Membranes: A Review of Membrane Wetting. Energy Fuels 2018, 32, 963–978.
- Witek-Krowiak, A.; Dawiec, A.; Modelski, S.; Podstawczyk, D. Carbon Dioxide Removal in a Membrane Contactor—Selection of Absorptive Liquid/Membrane System. Int. J. Chem. Eng. Appl. 2012, 3, 391–395.
- Zare, A.; Perna, L.; Nogalska, A.; Ambrogi, V.; Cerruti, P.; Tylkowski, B.; García-Valls, R.; Giamberini, M. Polymer Blends for Improved CO2 Capture Membranes. Polymers 2019, 11, 1662.
- Yong, J.K.J.; Cui, J.; Cho, K.L.; Stevens, G.W.; Caruso, F.; Kentish, S.E. Surface Engineering of Polypropylene Membranes with Carbonic Anhydrase-Loaded Mesoporous Silica Nanoparticles for Improved Carbon Dioxide Hydration. Langmuir 2015, 31, 6211–6219.
- Kim, T.J.; Lang, A.; Chikukwa, A.; Sheridan, E.; Dahl, P.I.; Leimbrink, M.; Skiborowski, M.; Roubroeks, J. Enzyme Carbonic Anhydrase Accelerated CO2 Absorption in Membrane Contactor. Energy Procedia 2017, 114, 17–24.
- RASOULI, H.; ILIUTA, I.; BOUGIE, F.; GARNIER, A.; ILIUTA, M.C. Hybrid Enzymatic CO2 Capture Process in Intensified Flat Sheet Membrane Contactors with Immobilized Carbonic Anhydrase. Sep. Purif. Technol. 2022, 287, 120505.
- Nguyen, K.; Iliuta, I.; Bougie, F.; Pasquier, L.C.; Iliuta, M.C. Techno-Economic Assessment of Enzymatic CO2 Capture in Hollow Fiber Membrane Contactors with Immobilized Carbonic Anhydrase. Sep. Purif. Technol. 2023, 307, 122702.
- Kurayama, F.; Matsuyama, T.; Yamamoto, H. Kinetic Study of a New Photosynthesis Bioreactor Design Using TiO2 Particles Combined with Enzymes. Adv. Powder Technol. 2005, 16, 517–533.
- Sun, J.; Wei, L.; Wang, Y.; Zhao, Z.; Liu, W. Immobilization of Carbonic Anhydrase on Polyvinylidene Fluoride Membranes. Biotechnol. Appl. Biochem. 2018, 65, 362–371.
- Sun, J.; Wang, C.; Wang, Y.; Ji, S.; Liu, W. Immobilization of Carbonic Anhydrase on Polyethylenimine/Dopamine Codeposited Membranes. J. Appl. Polym. Sci. 2019, 136, 1–9.
- Ren, S.; Li, C.; Tan, Z.; Hou, Y.; Jia, S.; Cui, J. Carbonic Hydrogel Composite Membrane with Improved Recycling and Stability for Efficient CO2 Capture. J. Agric. Food Chem. 2019, 67, 3372–3379.
- Wen, H.; Zhang, L.; Du, Y.; Wang, Z.; Jiang, Y.; Bian, H.; Cui, J.; Jia, S. Bimetal Based Inorganic-Carbonic Anhydrase Hybrid Hydrogel Membrane for CO2 Capture. J. CO2 Util. 2020, 39, 101171.
- Gundersen, M.T.; Gladis, A.; Fosbøl, P.L.; Von Solms, N.; Woodley, J.M. Operating Considerations of Ultrafiltration in Enzyme Enhanced Carbon Capture. Energy Procedia 2017, 114, 735–743.
- Guo, M.; Gu, F.; Meng, L.; Liao, Q.; Meng, Z.; Liu, W. Synthesis of Formaldehyde from CO2 Catalyzed by the Coupled Photo-Enzyme System. Sep. Purif. Technol. 2022, 286, 120480.
- Wang, Y.Z.; Zhao, Z.P.; Li, M.F.; Chen, Y.Z.; Liu, W. fang Development of a Hollow Fiber Membrane Micro-Reactor for Biocatalytic Production of Formate from CO2. J. Memb. Sci. 2016, 514, 44–52.
- Zhang, Y.; Liu, J. Bioinspired Photocatalytic NADH Regeneration by Covalently Metalated Carbon Nitride for Enhanced CO2 Reduction. Chem.–Eur. J. 2022, 28, e202201430.
More
