You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 2 by Beatrix Zheng.
Among the various materials used for the fabrication of nanoparticles, polycaprolactone (PCL) has gained considerable attention due to its biodegradability, biocompatibility, and ease of synthesis.
- self-assembly
- drug delivery
- vesicles
- multi-arm polymers
1. Introduction
Multi-arm architectures based on polycaprolactone (PCL) have been explored in recent years as a strategy to enhance the drug delivery efficiency of nanoparticles [1][2]. The multi-arm architecture offers several advantages over linear and dendritic polymers, such as increased stability, improved drug loading, and enhanced cellular uptake [3]. Several studies have reported the successful use of multi-arm PCL-based nanoparticles for the delivery of various drugs, including anticancer agents, antibiotics, and anti-inflammatory agents [4][5].Significant progress has been made in understanding the nanocarrier–architecture relationship [6][7][8], which can facilitate the development of stable formulations with enhanced drug bioavailability and safety. The variation of characteristics depending on architecture contributes to a growing interest of the scientific community towards these macromolecules. An analysis of the literature shows that the field has undergone a major expansion from a small group to a growing list of researchers, as reflected by the number of publications in recent years (Figure 1).
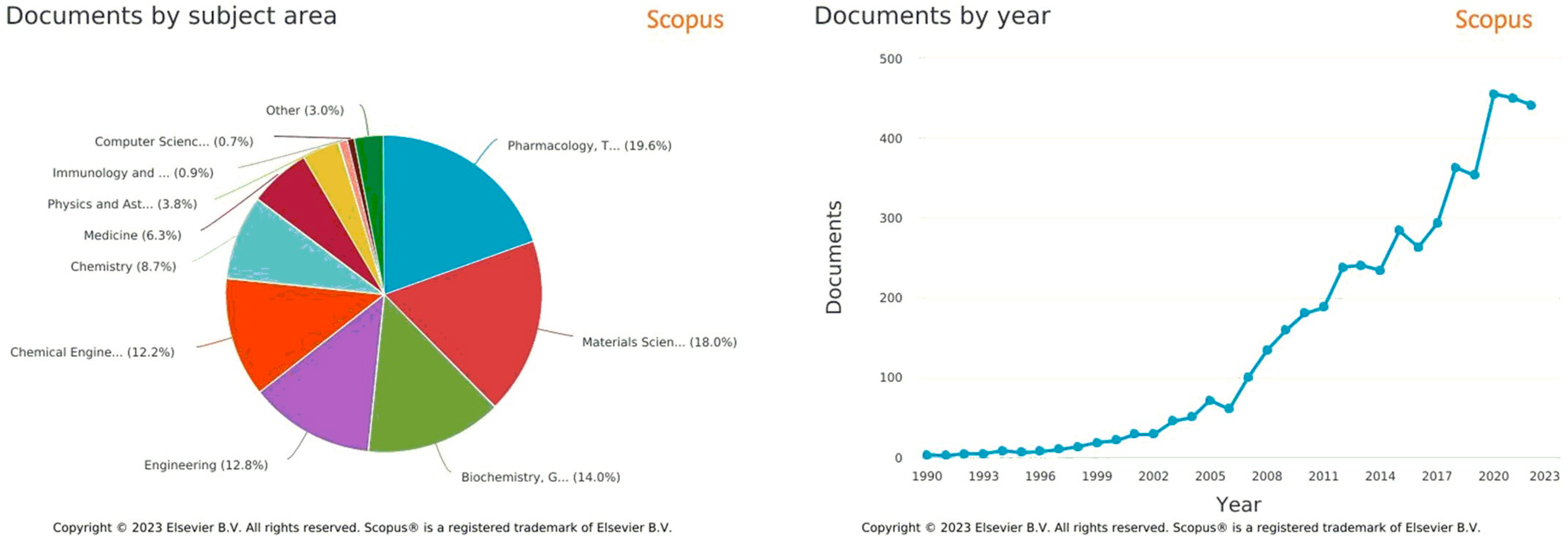
Figure 1. Bibliographic metrics from Scopus (https://www.scopus.com/, accessed on 26 February 2023), with “polycaprolactone multi arms for drug delivery” keywords, from 1990 to 2022.
2. Application of PCL-Based Materials in Nanoformulations
One of the main goals of nanocarrier-assisted drug administration is to improve the quality of life of patients through effective therapeutic interventions while seeking better management and treatment of high-risk and high-morbidity diseases in a broader perspective. Oral, injection, or inhalation absorption does not meet these requirements. This is why controlled release systems have been developed to address the targeting problems posed by traditional administration methods. In this case, different formulations of sustained-release systems can be used to treat ocular diseases, such as gels, nanoparticles, microparticles, or implants. This requires a very comprehensive in vitro and in vivo biological evaluation of these nanomedicines to determine key parameters such as safety, distribution, specificity, accumulation, and elimination, which would help pave the way for clinical implementation. This section provides a brief overview of efforts in this direction, specifically focusing on the concept of PCL-based nanovectors. The process of clinical translation is quite complex, and the challenges can only be overcome through continuous and elaborate studies of various PCL-based polymer formulations, both in vitro and in vivo. These studies will provide necessary information regarding key physicochemical parameters, the structure/efficacy relationship, and the safety of star polymers and their final products after degradation or metabolism. The efficacy of nanoparticles is strongly related to their ability to reach targeted sites in the body, overcoming the enormous challenges of sustained blood circulation and cellular/intracellular absorption.
Several studies in this field have provided a detailed overview of the factors that contribute to the improvement of the biological effects of nanocarriers. Upon introduction into the bloodstream, nanoparticles must be eliminated by the RES. In this case, previous research has shown that sustained-release systems are more effective in delivering drugs to targeted areas in the body, reducing the frequency of administration, decreasing toxicity, and improving patient compliance with treatment.
The problem of eliminating PCL-based materials, which have very slow degradation times by RES, has generally been solved by controlling opsonization, which depends on the physicochemical properties of nanoparticles. Despite this degradation problem, PCL has still been one of the most studied and applied biodegradable and biocompatible polymers in sustained-release systems. Additionally, PCL exhibits excellent mechanical and physical characteristics, as well as ease of processing and shaping at low temperatures. PCL has also been used in implantable devices for the sustained administration of various drugs such as antibiotics; antipsychotics; antiplatelet agents, namely dipyridamole and acetylsalicylic acid; nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen; or even thyroid hormones such as levothyroxine. Another advantage of PCL is its compatibility with a wide range of drugs, allowing for the homogeneous distribution of lipophilic drugs in the support matrix due to its hydrophobic nature [9][10].
It has been shown that PCL does not cause any notable inflammation as it does not produce acidic products from biodegradation. The degradation mechanism of PCL follows the random cleavage of the ester chain group by hydrolysis and can take up to 2–4 years, depending on the molecular weight of the PCL. The higher the molecular weight, the longer the natural decomposition of PCL.
PCL-based materials have been studied for in vitro and in vivo biocompatibility and efficacy, and several PCL drug delivery devices have been approved by the FDA. When reviewing the biological and pharmaceutical literature that introduces PCL as a base material, in most cases PEG is present, which is commonly used as a crown for nanoparticles in aqueous environments. PCL-based polymers, essentially those combined with PEG, have been the subject of several studies dealing with the contribution of these nanoformulation polymers to better therapeutic efficacy and their translation into preclinical and clinical trials.
As already emphasized, different forms of PCL-based delivery systems, such as microparticles and electrospun mats, films, and scaffolds, have been developed to improve in vivo and in vitro effects.
2.1. Electrospun Mats, Films and Scaffolds
Fibers loaded with drugs were designed to deliver the drug to the targeted site in a sustainable manner without any burst release. Thanks to their fibrous structure, electrospun mats have a larger surface area than films cast in a solvent. Therefore, larger amounts of drugs can be delivered to the site where the mats are implanted. This was demonstrated in F. Annuryanti’s study [11], in which Triamcinolone acetonide (TA) was loaded into PCL-based implants using 3D printing techniques, which avoided the use of organic solvents. The development of biodegradable ocular implants capable of providing prolonged release of a corticosteroid drug, such as TA, for at least 6 months is a major advance in the fight against posterior segment eye diseases such as diabetic retinopathy. Drugs such as decorin (proteoglycan), mefoxin, and sodium cefoxitin retained their functionality after the electrospinning process [12][13]. Nanofibers of PCL encapsulated in ampicillin and coated with PCL have been developed using a diluted and partially electrospinnable PCL solution as the shell and a fully electrospinnable PCL solution containing a drug as the core. In another study described by Poornima et al., coaxial electrospun core–shell chitosan-PCL nanofibers containing folic acid (anti-inflammatory) and resveratrol (pro-angiogenic) were prepared for wound healing applications. Star-shaped 6-arm copolymers (PCL-PEG) were synthesized with the aim of producing fibers that can be used in various applications such as clothing, medicine, sensors, and the automotive industry. The synthesis was performed with a discrete dipentaerythritol core that allows for the ROP of CL, followed by a Steglich esterification to attach PEG as the arms of this copolymer. To produce star-shaped block copolymer fibers, the electrospinning process was carried out under ambient conditions. The mechanical characteristics of the produced samples showed uniform and unique properties for micro-diameter fibers, which increases the fibers’ ability to trap drugs and can be used in clinical applications [14]. According to these results, poly (caprolactone) (PCL) has shown remarkable effectiveness for use as a biomaterial in scaffolds intended for tissue engineering, as well as for absorbable sutures, dressings, and anti-adhesion barriers, because it can adapt to different production methods. Materials containing PCL can be electrospun for the preparation of mats and can be used in various medical applications, including drug delivery [14]. Hsu et al. prepared electrospun PCL mats loaded with dexamethasone, which is used in the treatment of inner ear diseases and posterior segment eye diseases. Fibers with an average diameter of 300 nm and 670 nm were obtained. These hybrid systems allowed for the sustained release of dexamethasone for about one month [15]. In some cases, it is essential to modify the surfaces of fibers to improve drug attachment to nanofibers. For instance, to electrostatically bind doxorubicin to PCL, electrospun mats were partially hydrolyzed. Doxorubicin binding was achieved by immersing the hydrolyzed mats in drug solutions of different pH values. Drug absorption was high in basic medium, and drug release was high in acidic medium, indicating increased affinity for the tumor area [16]. In order to prevent rapid initial release under in vivo conditions, drugs were loaded into the core of coaxially electrospun nanofibers with a shell. Electrospinning can also be used to disperse particles and inorganic antimicrobial agents onto and into the surface of polymer fibers. When combined with clothing, these fibers can exhibit antibacterial properties. Therefore, electrospinning is promising for the development of membranes and fibers for protective clothing. Additionally, functional and intelligent fabrics can be made by blending two different materials and electrospinning composite fibers. These fibers can then be combined with a clothing membrane to create effective and practical materials. The fibers can be electrospun directly onto the fabric. Although this process overcomes the difficulties associated with sewing the electrospun membrane onto the fabric, the adhesion between the membrane and the fabric remains problematic. Several research organizations are studying plasma treatment strategies and chemical additives to improve membrane-to-fabric adhesion.2.2. Hydrogels
Hydrogels are three-dimensional networks of hydrophilic polymer chains that can swell in hydrophilic environments without losing their structure and cannot dissolve in organic and inorganic solvents due to the presence of cross-linking between the polymer chains. From a rheological point of view, hydrogels exhibit viscoelastic behavior and partially purely elastic behavior [1]. In another aspect, the hydrogel has a very porous structure that permits substrates to be loaded into the gel framework and released at a rate that depends on the diffusion coefficient of the substrate through the gel network. In this concept different types of PCL-based multi-arm copolymers have been synthesized and used as physical hydrogels with different thermosensitive characteristics. Multi-arm copolymers have been synthesized mainly by using a diol-initiated method such as PEG (with different numbers of arms). In this case, multi-block PEG/PCL copolymers with 3, 4, and 8 arms were synthesized using this method. At a certain concentration of the copolymer in the aqueous solution, the number of aggregates increases, and the volumetric fraction of the aggregates exceeds the maximum packing fraction, which leads to gelation of the system. Research has shown that the solubility of multi-arm copolymers in an aqueous medium is higher than that of linear polymers with the same hydrophobic/hydrophilic ratio. Additionally, PCL-based multi-arm copolymers form homogeneous and transparent hydrogels, whereas hydrogels formed from linear copolymers are generally white, opaque, and brittle, reducing their consistency during handling and limiting their applications in the field of encapsulation [17]. What is more impressive is that an increase in the number of PCL arms in the copolymers facilitates the aggregation and inter-aggregation of the bridges, thus facilitating the formation of the hydrogel [18]. The structure of PCL-based copolymers can influence the properties of the gel formed. PEG/PCL copolymers can be formed by an ester linkage between the PEG and PCL chains (PEG-OCO-PCL). The work of Buwalda and colleagues [19], who synthesized PEG-PCL copolymers with an amide bond between the chains (PEG-NHCO-PCL), showed that PEG-NHCO-PCL hydrogels exhibited a higher storage modulus (13 Kpa) than conventional hydrogels that contain ester bonds between the PEG-OCO-PCL chains (1.6 Kpa). This is due to the restricted conformational freedom of the amide groups, which leads to more effective physical cross-linking with a more rigid structure. In addition, interactions between the amide groups have caused an increase in the density of cross-linking and have improved the mechanical properties. In vitro studies have shown that the hydrolysis of PEG-OCO-PCL generally occurs at the ester linkages between PEG and PCL, resulting in a decrease in the hydrophobic chains of PCL and a disruption of the network structure. On the other hand, the amide groups in PEG-NHCO-PCL have been found to be more stable, and hydrolysis only occurs at the ester groups in the PCL chains. Thus, hydrogels formed from PEG-NHCO-PCL copolymers have greater in vitro stability (up to 16 days) than PEG-OCO-PCL hydrogels. Despite these advantages, hydrogels have several limitations. Significant swelling of the hydrogel in aqueous environments leads to the formation of materials with poor mechanical properties that limit their use in load-bearing applications. Hydrophobic substrates such as drugs cannot be applied to hydrogels due to the thermodynamic incompatibility between the hydrophilic chain, water, and the hydrophobic drug.2.3. Nanoparticles
Colloidal systems based on PCL are of great interest due to their use as drug carriers [20]. Self-assembled structures (micelles) have been widely studied as encapsulation agents for various drugs and proteins [21]. The use of nanocarriers for drug delivery arose from the need to find new vehicles for bioactive agents that would provide pharmacokinetic profiles that mimic the normal pattern of these agents, when the therapeutic efficacy of many of these agents is limited by the therapeutic index and selectivity [22]. Encapsulation methods improve and prolong the stability of bioactive drugs, enhance therapeutic efficacy by adapting the precise amount of drug to achieve the desired therapeutic response, and prevent degradation and nonspecific uptake by cells, thus minimizing side effects [23]. The structure of a transport system has a major impact on the loading of bioactive drugs and affects their release, cellular internalization, and in vivo biodistribution [24]. Micellar structures and micro- or nanoparticles of different morphologies allow for better control of degradation rates, release behavior, or drug distribution in the body [25]. An analysis of the literature, as well as these examples, show that a large part of the evaluation of multi-armed PCL-based polymers in nanoparticle formulation design has focused on drug loading and release, as well as in vitro studies on different cell lines, generally related to cancerous tissues. Some in vivo studies have also been conducted, but they remain few in comparison. For example, in amphiphilic polymer-based multi-armed micelles with dimensions of 10–100 nm, they are known to reduce protein-nanoparticle interactions and avoid nanocarrier elimination by phagocytosis [26]. To limit RES-based elimination and improve blood retention and circulation, copolymers can bind to drug molecules to increase their molecular weight, thereby reducing their elimination kinetics and increasing their effectiveness. The nanoparticles then follow passive pathways (enhanced retention by permeation) or active pathways (using targeting motifs) to reach the extracellular space. Accumulation at tumor sites depends on the camouflage, stability, blood circulation/retention, targeting ability of the nanoparticles, and other factors such as vascularization and angiogenesis [27]. Nanoformulations using multi-arm amphiphilic polymers help solve these RES-based elimination problems by modulating the density of the nanoparticle corona and facilitating the introduction of functional groups for active transport and drug release in response to stimuli. Cellular internalization of nanoparticles can occur through specific interactions with receptors, endocytosis, nonspecific association with the cell membrane, and absorption by pinocytosis [28]. Thanks to controlled and targeted drug efficiency, as well as their great ability to cross physiological barriers, biodegradable polymer nanoparticle-based delivery systems have shown sustained drug activity. Additionally, in addition to their reduced systemic side effects, drug-loaded nanoparticle systems reduce costs for patients and risks of toxicity. In this case, particle size is the primary parameter that influences targeting capabilities and penetration through physiological barriers. PCL-based nanoparticles have generally been prepared using several techniques such as solvent evaporation-emulsification, nano precipitation, and solvent displacement methods, which provide richness in the operating modes used. During nanoparticle preparation, several parameters must be optimized, such as drug–polymer concentrations, homogenization speed and time, and types of solvents used. The effect of these parameters on the properties of curcumin-loaded PCL nanoparticles has been studied in the work of Kasinathan et al. [29]. In this research, PCL nanoparticles loaded with curcumin (with a diameter of about 390 nm) were prepared using the solvent emulsification and evaporation technique. The solvent emulsion-evaporation method was also used for intranasal brain delivery. PCL nanoparticles (about 300 nm) loaded with carboplatin (hydrophilic chemotherapy) were prepared for this purpose. In vitro cytotoxicity tests against human glioblastoma LN229 cells and in situ nasal perfusion tests conducted on rats showed better results compared to the use of a simple drug solution. The RPE effect has become the most important mechanism considered in the design of cancer therapy. Nanocarriers are designed to take advantage of this RPE effect and accumulate in the tumor environment for better targeting and therapeutic efficacy. Vascularized, mainly metastatic, tumors of the pancreas, prostate, and liver are less sensitive to the RPE effect than other tumor types [30]. PCL-based micelles have multiple advantages, including improved permeation and retention (EPR), increased blood circulation time, and increased endocytosis due to surface modification. For the treatment of hepatocellular carcinoma, Chen and colleagues prepared lipiodol-loaded micelles using polyethylene glycol-polycaprolactone (PEG-PCL) copolymers and amphiphilic hyaluronic acid-polycaprolactone (HA-g-PCL) copolymers [31]. The HA-g-PCL micelles showed high drug encapsulation efficiency (about 71%), stability in aqueous solutions, and high affinity and cytotoxicity towards HepG2 cells, despite their size of 274 nm. The combination of drugs of interest with PCL-based formulations allows for non-covalent encapsulation of these drugs (based on hydrogen bonding interactions, hydrophobic-hydrophobic interactions, or ionic interactions) [32]. In this case, several factors such as drug solubility, drug affinity for the polymer, nanoparticle core volume, and drug self-aggregation capacity can influence the drug loading capacity (DLC), which is the mass ratio of drug to polymer. The DLC of paclitaxel in PEG-b-PCL-based micelles, for example, increases with the length of the hydrophobic PCL block [33]. On the other hand, no significant increase was observed when a similar experiment was conducted with doxorubicin, a less hydrophobic compound [34]. Furthermore, studies on the effectiveness of administration using in vitro cytotoxicity studies have also indicated that longer hydrophilic arms compromise the therapeutic efficacy of star-shaped supramolecular micelles charged with DOX, resulting in significantly reduced cytotoxicity, as measured by a higher IC50 value [35]. In addition to several experimental approaches, physicochemical models have been used to predict the charge and stability of the drug based on compatibility parameters between the polymer and the drug [36]. Among these models is the Flory–Huggins interaction parameter (ΧFH), which has been successfully used to characterize polymer-drug compatibilities [37]. Studies on PCL-based models have shown that drug loading is strongly influenced by the polymer composition and the shape of the nanoparticles [38]. “Worm-like” structures exhibit a higher packing density of PEG halves on surfaces with reduced curvature, leading to higher packing constraints for the polymers. Under these conditions, PEG chains extend, thus increasing the thickness of the outer PEG layer. Consequently, "worm-like" structures exhibit a drug loading twice that of spherical NPs. This variety of methods in the field of nanoparticles can facilitate screening of formulations and be used for the rational design of new polymers and drug-specific nanocarriers. It should be noted that the combination of a drug with a specific nanocarrier can have a significant influence on the pharmacokinetic profile and pharmacological effect of the drug [39]. “Intelligent” stimulus-sensitive nanoparticles could improve therapeutic effectiveness by enabling controlled drug delivery to the site of action. These formulations can be activated by external or internal stimuli, such as pH changes, temperature, irradiation, magnetism, concentration gradients, or enzymatic activity. Most cancer cells are found in an acidic environment, which stimulates the development of pH-sensitive drug delivery systems. This helps to reduce the harmful side effects of drugs used to treat cancer. Systems have been developed to respond to these stimuli and increase drug delivery to the target site [1]. Qi et al. prepared pH-sensitive copolymer micelles. These micelles, which measure approximately 100 nm, were obtained from a diblock copolymer of PEG and PCL linked by a pH-sensitive hydrazine (Hyd) bond, using a solvent evaporation method. The micelles were loaded with Paclitaxel (PTX), an anticancer drug. The results showed that these pH-sensitive and biodegradable copolymer micelles containing hydrazine exhibited strong toxicity against HepG2 cells [40]. In a different study, poly(ε-caprolactone) micelles grafted onto guar gum (GG-g-PCL) were prepared using a membrane dialysis method to load ketoprofen. Approximately 18% by weight of the drug was loaded into micelles ranging in size from 75 to 162 nm. Drug release was characterized by an initial rapid release, followed by sustained release over a period ranging from 10 to 68 hours [41]. Davoodi et al. [42] created amphiphilic graft copolymer systems of polyethyleneimine (PEI)-polycaprolactone (PCL) by combining non-viral gene therapy drugs with chemotherapy drugs to be used against aggressive cancers. They encapsulated the anticancer drug doxorubicin (Dox) and p53 plasmid DNA in positively charged nanoparticles (about 159 nm) with a hydrophobic core and a hydrophilic shell. The synergistic effect of Dox and p53 plasmid DNA showed better cytotoxic efficacy against human hepatoma and human cervical adenocarcinoma cell lines compared to their single-agent counterparts. In another study, linear, 3-arm, and 6-arm star-shaped PCL-poly(quaternary ammonium salt) copolymers were synthesized using a combination of ring-opening polymerization and atom transfer polymerization. The hydrophobic PCL block formed the core, while the hydrophilic poly(quaternary ammonium salt) blocks served as the shell of the micelle. The antibacterial drug triclosan was loaded into these solvent-evaporated micelles. The efficacy of all types of micelles against Escherichia coli was reported, with star-shaped micelles showing better antibacterial properties [14]. Amphiphilic micelles of a multi-armed copolymer based on PCL/PEG and cyclodextrin developed by Xiufang Li et al. [43], have shown great efficiency in encapsulating DOX due to the adequate space present in this system. The in vitro release of the drug was studied using electrochemical control. In vitro release studies of DOX from star-shaped copolymeric micelles have shown that this anticancer drug can be released over several hours. The ability of multi-armed copolymers to load and encapsulate a large number of drugs compared to linear analogues has been demonstrated in several other studies that have addressed drug delivery applications (Table 1) [44].Table 1. The Biological Evaluations of PCL-based materials.
| Mode | Polymers | Drug | Nanoformulation | Therapeutic Target | Biological Evaluation | Ref. |
|---|---|---|---|---|---|---|
| Electrospun | PCL | Dexamethasone | Nanofibers | Biomedical applications, particularly in the eye. | Although the results are encouraging, further in vitro studies and finally in vivo animal studies are needed to determine the comfort and retention. | [45] |
| Electrospun | PCL | Tetracycline/βcyclodextrin | Nanofibers | Controlled release systems and their clinical application. | Strong adhesion and reduced demineralization of dentin. | [46] |
| High antimicrobial activity against A.a and P.g. | ||||||
| Electrospun | PCL | ampicillin | Nanofibers | Zero-order drug release kinetics. | [47] | |
| Electrospun | PCL | Seeded with IPFP and chondrones | Nanofibers | Restore the functional cartilage in articular disorders. | A positive effect on the differentiation of IPFP APCs into chondrogenic cells. | [48] |
| Electrospun | PCL-PEG | Fe3O4 NPs | Magnetic composite membrane (PCEC/Fe3O4 nanofibers) | Preventing tumor recurrence and improving dermal wound healing after an excision of malignant tumor in the skin. | In vitro cell culture of NIH 3T3 cells on the PCEC/Fe3O4 membranes showed that the PCEC/Fe3O4 fibers might be a suitable scaffold for cell adhesion. | [49] |
| MTT analysis also demonstrated that the membranes possessed lower cytotoxicity. | ||||||
| Electrospun | PCL-PEG | PCL fibers embedded in a PEG-fibrinogen hydrogel | Sufficient cell-approachable bio-signaling cues, which may synergistically facilitate the control of stem cell fates for regenerative therapies. | A novel nanocomposite that promoted the active interactions with stem cells and exerted excellent fibrogenic commitment in vitro. | [50] | |
| Hydrogel films | PCL-PEG | Curcumin | Sol-gel | Safe candidate for in situ gel-forming controlled drug delivery system. | No toxic response or histopathological changes were observed. | [51] |
| In vivo gel-formation, degradation test showed that a complete degradation occurred after 21 days. | ||||||
| Hydrogel films | PCL-PEG | proteins (BSA and HRP) | Sol-gel | In-situ gel depot at body temperature providing drug release control. | The in vivo gel-formation and degradation studies indicated that copolymer hydrogels can sustain at least 45 days. | [52] |
| Hydrogel films | PCL-PEG | Sol-gel | Facilitate the bone regeneration in the non-load-bearing cranial repair process by combining the advantages of the intrinsic osteoinductive ABM granules and the injectable thermosensitive PECE hydrogel. | In vivo bone regeneration performance was carried out in white rabbits for 4, 12, and 20 weeks. | [53] | |
| Hydrogel films | (PEG-PCL)3 | cyclodextrin | Injectable hydrogels | Treatment of joint disease. | In vitro drug release showed that DOX∙HCl was released in a controlled, pH-dependent manner. | [54] |
| Nanoparticles | β-cyclodextrin-PCL | Camptothecin/DOX | Micelles | Sustained release of hydrophobic drugs via local administration in clinical situations | IND-M was able to release the drug over an extended period in vitro and exhibited a significant therapeutic effect in pharmacodynamic studies in vivo (HepG2 cells). | [55] |
| Nanoparticles | 12-arm PEG-PCL | Docetaxel | Micelles | In vitro cytotoxicity (HeLa cells) study indicated a reduced cytotoxicity. | [35] |
References
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60.
- Behl, A.; Parmar, V.S.; Malhotra, S.; Chhillar, A.K. Biodegradable diblock copolymeric PEG-PCL nanoparticles: Synthesis, characterization and applications as anticancer drug delivery agents. Polymer 2020, 207, 122901.
- Baghbanbashi, M.; Kakkar, A. Polymersomes: Soft Nanoparticles from Miktoarm Stars for Applications in Drug Delivery. Mol. Pharm. 2022, 19, 1687–1703.
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063.
- Lotocki, V.; Kakkar, A. Miktoarm star polymers: Branched architectures in drug delivery. Pharmaceutics 2020, 12, 827.
- Villemin, E.; Ong, Y.C.; Thomas, C.M.; Gasser, G. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat. Rev. Chem. 2019, 3, 261–282.
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718.
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253.
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23.
- Wang, X.; Wang, Y.; Wei, K.; Zhao, N.; Zhang, S.; Chen, J. Drug distribution within poly (ɛ-caprolactone) microspheres and in vitro release. J. Mater. Process. Technol. 2009, 209, 348–354.
- Annuryanti, F.; Domínguez-Robles, J.; Anjani, Q.K.; Adrianto, M.F.; Larrañeta, E.; Thakur, R.R.S. Fabrication and Characterisation of 3D-Printed Triamcinolone Acetonide-Loaded Polycaprolactone-Based Ocular Implants. Pharmaceutics 2023, 15, 243.
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials-Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269.
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56.
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893.
- Hsu, K.H.; Fang, S.P.; Lin, C.L.; Liao, Y.-S.; Yoon, Y.-K.; Chauhan, A. Hybrid electrospun polycaprolactone mats consisting of nanofibers and microbeads for extended release of dexamethasone. Pharm. Res. 2016, 33, 1509–1516.
- Jassal, M.; Sengupta, S.; Bhowmick, S. Functionalization of electrospun poly (caprolactone) fibers for pH-controlled delivery of doxorubicin hydrochloride. J. Biomater. Sci. Polym. Ed. 2015, 26, 1425–1438.
- Lu, C.; Liu, L.; Guo, S.R.; Zhang, Y.; Li, Z.; Gu, J. Micellization and gelation of aqueous solutions of star-shaped PEG–PCL block copolymers consisting of branched 4-arm poly (ethylene glycol) and polycaprolactone blocks. Eur. Polym. J. 2007, 43, 1857–1865.
- Lu, C.; Guo, S.r.; Zhang, Y.; Yin, M. Synthesis and aggregation behavior of four types of different shaped PCL-PEG block copolymers. Polym. Int. 2006, 55, 694–700.
- Buwalda, S.J.; Nottelet, B.; Coudane, J. Robust & thermosensitive poly (ethylene glycol)-poly (ε-caprolactone) star block copolymer hydrogels. Polym. Degrad. Stab. 2017, 137, 173–183.
- Jeong, J.C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly (ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258.
- Ahmed, F.; Discher, D. Controlled release from polymersome vesicles blended with PEO-PLA or related hydrolysable copolymer. J. Control. Release 2004, 96, 37–53.
- Aliabadi, H.M.; Mahmud, A.; Sharifabadi, A.D.; Lavasanifar, A. Micelles of methoxy poly (ethylene oxide)-b-poly (ɛ-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J. Control. Release 2005, 104, 301–311.
- Park, E.K.; Kim, S.Y.; Lee, S.B.; Lee, Y.M. Folate-conjugated methoxy poly (ethylene glycol)/poly (ɛ-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. J. Control. Release 2005, 109, 158–168.
- Quaglia, F.; Ostacolo, L.; De Rosa, G.; La Rotonda, M.I.; Ammendola, M.; Nese, G.; Maglio, G.; Palumbo, R.; Vauthier, C. Nanoscopic core-shell drug carriers made of amphiphilic triblock and star-diblock copolymers. Int. J. Pharm. 2006, 324, 56–66.
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350.
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510.
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907.
- Li, J.; Mao, H.; Kawazoe, N.; Chen, G. Insight into the interactions between nanoparticles and cells. Biomater. Sci. 2017, 5, 173–189.
- Kasinathan, N.; Amirthalingam, M.; Reddy, N.D.; Jagani, H.V.; Volety, S.M.; Rao, J.V. In-situ implant containing PCL-curcumin nanoparticles developed using design of experiments. Drug Deliv. 2016, 23, 1007–1015.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403.
- Chen, S.C.; Yang, M.H.; Chung, T.W.; Jhuang, T.S.; Yang, J.D.; Chen, K.C.; Chen, W.J.; Huang, Y.F.; Jong, S.B.; Tsai, W.C. Preparation and characterization of hyaluronic acid-polycaprolactone copolymer micelles for the drug delivery of radioactive iodine-131 labeled lipiodol. BioMed Res. Int. 2017, 2017.
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release 2014, 193, 9–26.
- Khoee, S.; Hassanzadeh, S.; Goliaie, B. Effects of hydrophobic drug–polyesteric core interactions on drug loading and release properties of poly (ethylene glycol)–polyester–poly (ethylene glycol) triblock core–shell nanoparticles. Nanotechnology 2007, 18, 175602.
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly (ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426.
- Zuo, C.; Peng, J.; Cong, Y.; Dai, X.; Zhang, X.; Zhao, S.; Zhang, X.; Ma, L.; Wang, B.; Wei, H. Fabrication of supramolecular star-shaped amphiphilic copolymers for ROS-triggered drug release. J. Colloid Interface Sci. 2018, 514, 122–131.
- Huynh, L.; Neale, C.; Pomès, R.; Allen, C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 20–36.
- Nagarajan, R.; Barry, M.; Ruckenstein, E. Unusual selectivity in solubilization by block copolymer micelles. Langmuir 1986, 2, 210–215.
- Loverde, S.M.; Klein, M.L.; Discher, D.E. Nanoparticle shape improves delivery: Rational coarse grain molecular dynamics (rCG-MD) of taxol in worm-like PEG-PCL micelles. Adv. Mater. 2012, 24, 3823–3830.
- Cheng, F.; Guan, X.; Cao, H.; Su, T.; Cao, J.; Chen, Y.; Cai, M.; He, B.; Gu, Z.; Luo, X. Characteristic of core materials in polymeric micelles effect on their micellar properties studied by experimental and dpd simulation methods. Int. J. Pharm. 2015, 492, 152–160.
- Qi, P.; Bu, Y.; Xu, J.; Qin, B.; Luan, S.; Song, S. pH-responsive release of paclitaxel from hydrazone-containing biodegradable micelles. Colloid Polym. Sci. 2017, 295, 1–12.
- Tiwari, A.; Prabaharan, M. An amphiphilic nanocarrier based on guar gum-graft-poly (ε-caprolactone) for potential drug-delivery applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 937–949.
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination therapy of killing diseases by injectable hydrogels: From concept to medical applications. Adv. Healthc. Mater. 2021, 10, 2001571.
- Gong, C.Y.; Shi, S.; Peng, X.Y.; Kan, B.; Yang, L.; Huang, M.J.; Luo, F.; Zhao, X.; Wei, Y.-Q.; Qian, Z.-Y. Biodegradable thermosensitive injectable PEG-PCL-PEG hydrogel for bFGF antigen delivery to improve humoral immunity. Growth Factors 2009, 27, 377–383.
- Kost, B.; Brzeziński, M.; Socka, M.; Baśko, M.; Biela, T. Biocompatible polymers combined with cyclodextrins: Fascinating materials for drug delivery applications. Molecules 2020, 25, 3404.
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681.
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for Fast-Disintegrating oral drug delivery. J. Colloid Interface Sci. 2022, 610, 321–333.
- Wang, Y.; Yu, D.G.; Liu, Y.; Liu, Y.N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289.
- Nikpou, P.; Soleimani Rad, J.; Mohammad Nejad, D.; Samadi, N.; Roshangar, L.; Navali, A.M.; Shafaei, H.; Nozad Charoudeh, H.; Danandeh Oskoei, N.; Soleimani Rad, S. Indirect coculture of stem cells with fetal chondrons using PCL electrospun nanofiber scaffolds. Artif. Cells Nanomed. Biotechnol. 2017, 45, 283–290.
- Zhang, H.; Xia, J.; Pang, X.; Zhao, M.; Wang, B.; Yang, L.; Wan, H.; Wu, J.; Fu, S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2017, 73, 537–543.
- Nakajima, I.; Tsukimura, T.; Ono, T.; Shiga, T.; Shitara, H.; Togawa, T.; Sakuraba, H.; Miyaoka, Y. In Vivo Delivery of Therapeutic Molecules by Transplantation of Genome-Edited Induced Pluripotent Stem Cells. bioRxiv 2023, 2003, 522057.
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J. Mater. Sci. Mater. Med. 2019, 30, 1–11.
- Bonacucina, G.; Cespi, M.; Mencarelli, G.; Giorgioni, G.; Palmieri, G.F. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers 2011, 3, 779–811.
- Ni, P.; Ding, Q.; Fan, M.; Liao, J.; Qian, Z.; Luo, J.; Li, X.; Luo, F.; Yang, Z.; Wei, Y. Injectable thermosensitive PEG–PCL–PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 2014, 35, 236–248.
- Hu, J.; Zhang, M.; He, J.; Ni, P. Injectable hydrogels by inclusion complexation between a three-armed star copolymer (mPEG-acetal-PCL-acetal-) 3 and α-cyclodextrin for pH-triggered drug delivery. RSC Adv. 2016, 6, 40858–40868.
- Wei, X.; Lv, X.; Zhao, Q.; Qiu, L. Thermosensitive β-cyclodextrin modified poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) micelles prolong the anti-inflammatory effect of indomethacin following local injection. Acta Biomater. 2013, 9, 6953–6963.
More
