You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 4 by Dean Liu and Version 3 by Dean Liu.
An anti-corrosion inhibitor is one of the most useful methods to prevent metal corrosion toward different media. In comparison with small molecular inhibitors, a polymeric inhibitor can integrate more adsorption groups and generate a synergetic effect, which has been widely used in industry and become a hot topic in academic research.
- metal corrosion
- anti-corrosion inhibitor
- polymeric inhibitor
1. Phosphorus-Containing Synthetic Polymeric Inhibitor
Phosphorus can coordinate with metal surfaces more strongly than oxygen, which has been introduced to polymeric inhibitor as additional adsorption site in the form of both coating and solution. Generally, there are two kinds of strategies to prepare phosphorus-containing synthetic polymeric inhibitor: (i) post-modification of natural polymer [1]; and (ii) direct polymerization of phosphorus-containing monomer [2].
Grafting phosphorus-containing groups to natural polymers not only can introduce additional adsorption sites, but also improve their water solubility. David et al. functionalized chitosan with phosphonic acids (P-1 in Figure 1) via the Kabachnik-Fields reaction and subsequent hydrolysis [1]. The introduction of phosphonic acid groups greatly decreased the dynamic viscosity of chitosan. Moreover, compared with the native chitosan, P-1 showed improved adsorption on the carbon steel surface and better corrosion inhibition property. Meanwhile, such material can also be used in metallic aluminum corrosion protection. David et al. [1] fabricated inhibitive coating by the layer-by-layer (L-b-L) technique from native chitosan or synthesized phosphorylated chitosan (P-1 in Figure 1) combined with alginate functionalized chitosan. It was found that the coating can create a physical barrier that acts mainly by reducing the active surface area and has the effect of blocking the penetration of the aggressive species into the metal substrate. According to the result of electrochemical impedance spectroscopy (EIS) in 0.1 M Na2SO4 solution, the as prepared coating showed improved corrosion resistance for aluminum alloy 3003.
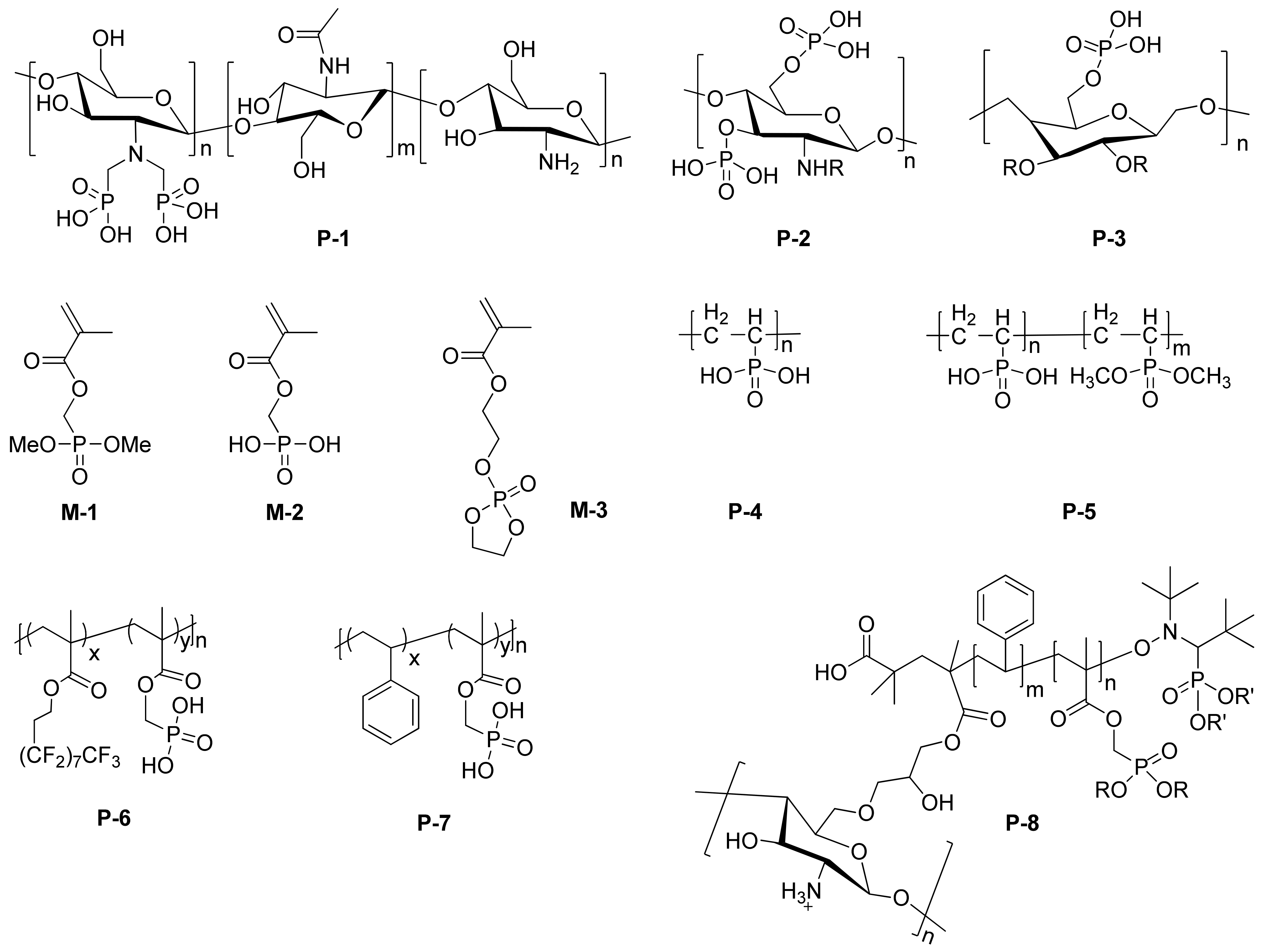
Figure 1. Chemical structures of typical phosphorus-containing polymeric inhibitors (P1–P8) and monomers (M1–M3).
Phosphonic acids can also be introduced to chitin. As demonstrated by Hebalkar and coworkers, they synthesized phosphorylated chitin (P-2 in Figure 1) to solve the water solubility of native chitin and improve their coordination ability with metal surface [3]. According to the gravimetric analysis in NaCl solution, only 200 ppm P-2 can protect copper very well with maximum inhibition efficiency as high as 92%.
By using simple phosphorylation, phosphonic acids can be introduced to ethyl cellulose. Ben Youcef et al. [4] reported the method of microcapsulation to encapsulate almond oil as inhibitor by using phosphorylated ethyl cellulose (P-3 in Figure 1). It was found that the P-O− functionalized groups strongly interacted with the metal ions in the metal substrate, and therefore, generated synergetic anti-corrosion effect for almond oil.
Comparing with the post-functionalization of natural polymer, the direct polymerization of phosphorus-containing monomer provides accurate structure control and rational design of polymeric inhibitors [5]. The most commonly used phosphorus-containing methacrylate-base monomers are shown in Figure 1 (M-1 and M-2). For example, Keil et al. [6] reported UV-cured polyester acrylate coatings on zinc and iron as anti-corrosion surface. Moreover, Pebere et al. [7] extended the study on the UV polymerization of M-1 and M-2 to prepare effective corrosion protection. It was found that coatings containing phosphonic acid methacrylate (M-2) showed better anti-corrosive performance than those containing methacrylate phosphonic dimethyl ester (M-1), owing to the improved adsorption of phosphonic acid to metal surface than corresponding ester. Similarly, Ilia et al. [8] copolymerized vinylphosphonic acid (VPA) and dimethyl vinylphosphonate (DMVP) to prepare PVPA-co-PDMVP copolymer P-5 (Figure 1) via free radical photopolymerization. Interestingly, they found that the presence of phosphonate groups from DMVP in copolymers was beneficial and a molar ratio VPA:DMVP 4:1 and 3:1 enhanced the anticorrosion for iron surface in comparison with homopolymer of vinylphosphonic acid (P-4). In other words, the coordination between P-OH and metal surface competes with the formation rate of uniform protective layer. While copolymers with VPA:DMVP 4:1 may show higher diffusion coefficient, and therefore, faster formation of protective film than other polymers.
In addition to introducing additional adsorption sites to polymeric inhibitors, low surface energy monomers have also been used in the design of efficient inhibitor. Moratti et al. [9] prepared block copolymer P-6 (Figure 1) via free radical polymerization of heptafluorodecyl methacrylate and (dimethoxyphosphoryl) methyl methacrylate. The copolymers were then immobilized as a monolayer film to the surface of 316L stainless steel by treatment of dilute solutions in trifluoroacetic acid for 30 min followed by rinsing. Owing to the presence of fluorinated block and the presence of adsorption site from the phosphorus block, the resulting polymeric inhibitor exhibited excellent anti-corrosion property and long-term stability.
Champagne et al. [2] reported the nitroxide-mediated polymerization (NMP) to prepared polystyrene-b-poly(dimethyl(methacryloyloxy)methyl phosphonate) and corresponding polystyrene-b poly(dimethyl(methacryloyloxy)methyl phosphonic acid) (P-7 in Figure 1) and its graft onto polysaccharide chitosan (P-8 in Figure 1). The controlled radical polymerization feature of NMP allowed rational design of the repeat unit and the topology of the resulting polymer. It can be anticipated that the resulting grafted polymers are potential candidate for excellent inhibitor due to the coexistence of chitosan and phosphorus-containing polymers (Table 1).
In addition to the radical-based polymerization, sol-gel method has also been developed for the preparation of phosphorus-containing polymeric inhibitors. Mandler et al. [10] incorporated phenylphosphonic acid (PPA) to a sol-gel film to enhance the corrosion protection of metallic aluminum; however, it was found that such method resulted in aggregation and phase separation. To overcome such problem, Choudhury et al. [11] proposed the design of networked methacrylate- hybrid by copolymerizing 2-(methacryloyloxy)ethyl phosphate (M-3 in Figure 1), containing a polymerizable methacrylate group and functional phosphate group with 3-[(methacryloyloxy)propyl] trimethoxysilane [12]. It was found that the proposed network not only enhanced the binding of the coating to the metal substrate via the acid-base interaction of the P-O− group of the phosphate with the Mn+ of the metal substrate, but also resulted excellent anti-corrosion property.
Table 1. Inhibition property of typical phosphorus-containing synthetic polymeric inhibitor.
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference |
|---|---|---|---|---|---|
| P-1 | aluminum alloy 3003 | pH = 5 acetic acid | EIS | / | [1] |
| P-2 | copper | 200 × 10−3 g/L NaCl | weight loss, EIS | 92 | [3] |
| P-3 | mild steel | 3.5% NaCl | salt spray test | / | [4] |
| M-1/M-2 | low-carbon steel | 0.1 M NaCl | EIS | 85 | [6] |
| M-3 | mild steel | 3.5% NaCl | PDP, EIS | [11] | |
| P-4/P-5 | iron coin | 3% NaCl | PDP, EIS | 83.5 | [8] |
| P-6 | 316L stainless steel | trifluoroacetic acid | long term stability tests | / | [9] |
2. Sulfur-Containing Synthetic Polymeric Inhibitor
As a classic coordination atom, sulfur can form a stable complex with different metal ions. Generally, there are two kinds of sulfur-containing polymeric inhibitors: (i) polythiophene and (ii) polysulfone. Normally, intrinsically conducting polymers or conjugated polymers, such as polythiophene, polypyrrole, and polyaniline, have been widely used as protective coating for corrosion protection of steel [13]. Polythiophene was first reported as corrosion protection in 1989 [14]. The facile electropolymerization of thiophene and its derivatives allows the preparation of homogeneous polymer films on the surface of different metals with good electrical properties and chemical stability [15]. Gonzalez-Rodriguez and coworkers [16] compared the anti-corrosion property of poly(3-octyl thiophene) (P3OT) and poly(3-hexylthiophene) (P3HT) (P-9 in Figure 2). The plate they used was a commercially available 1018 carbon steel sheets, and copper wires were welded to the plate, which was used as a reaction platform for electrode deposition. The polymer solution was then deposited on the electrode, evaporated solvent, dried, and annealed to afford the P3OT/P3HT coating. Both polymer films were found to be effective in protecting the substrate from corrosion by decreasing the critical current necessary to passive the substrate, increasing the pitting potential, and broadening the passive interval, and P3HT was found to be more effective due to a much lower number of defects than P3OT films. Interestingly, P3HT gave better protection than P3OT, because of the lower defects in the film of P3HT than that for the P3OT films. Thiophene can also be copolymerized with other monomers to improve the anti-corrosion property. For example, Branzoi et al. [17] investigated the anti-corrosive properties of poly(N-methylpyrrole-Tween20/3-methylthiophene) coatings on carbon steel type OLC 45 in 0.5 M H2SO4 medium. The surfactant Tween 20 was a dopant used in the electropolymerization process, which could improve the anti-corrosive properties by hindering the corrosive sulfate ion penetration. The corrosion rate of PNMPY-TW20/P3MT-coated OLC 45 has been indicated to be ~10 times reduced in comparison with uncoated OL 45, and the corrosion protection efficiency of the coating is above 90%. More importantly, the anti-corrosion property of such coating can be tuned by the condition of electropolymerization, such as electrodeposition current and time, highlighting the potential application of such technique.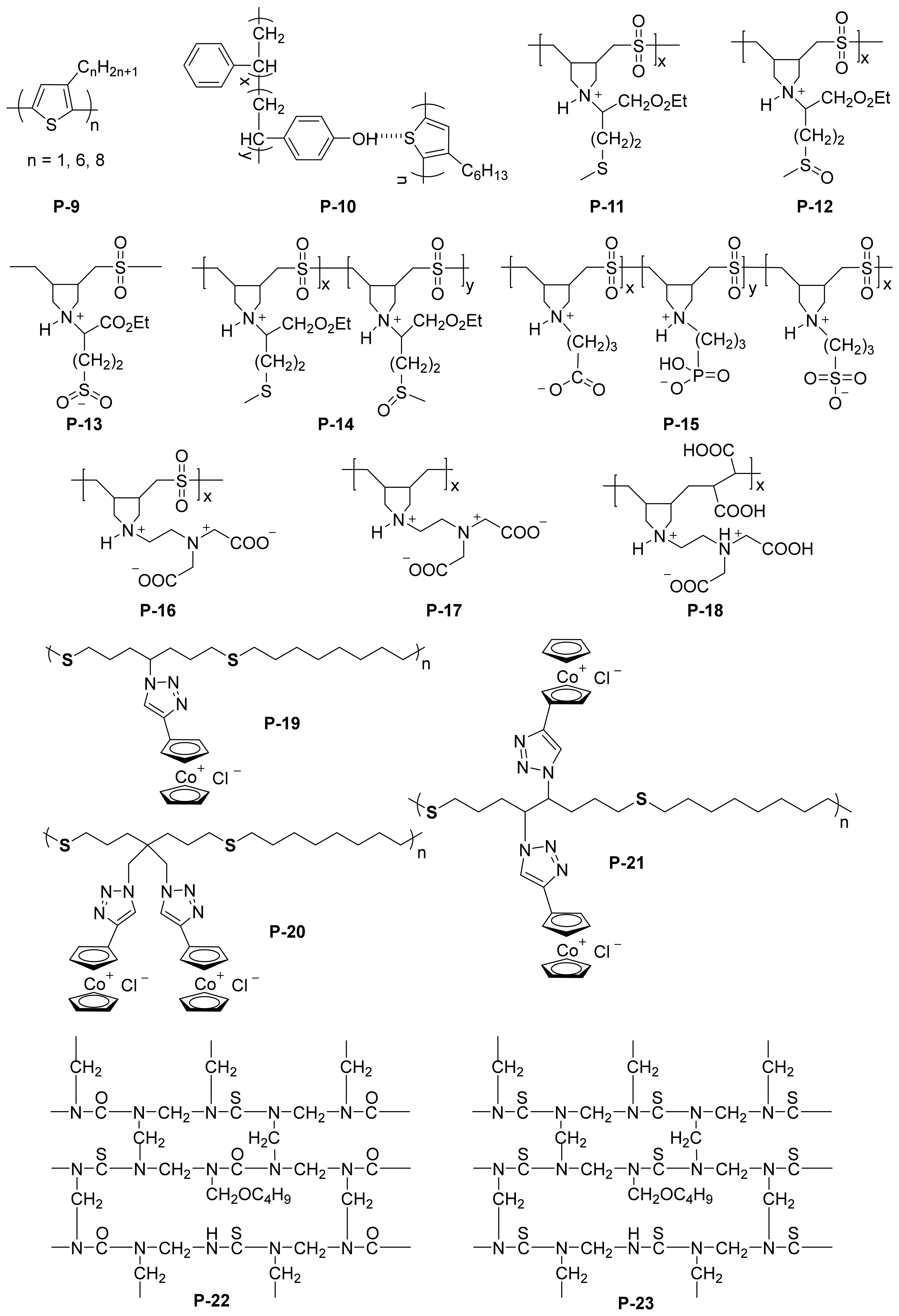
Figure 2. Chemical structures of typical sulfur-containing polymeric inhibitors.
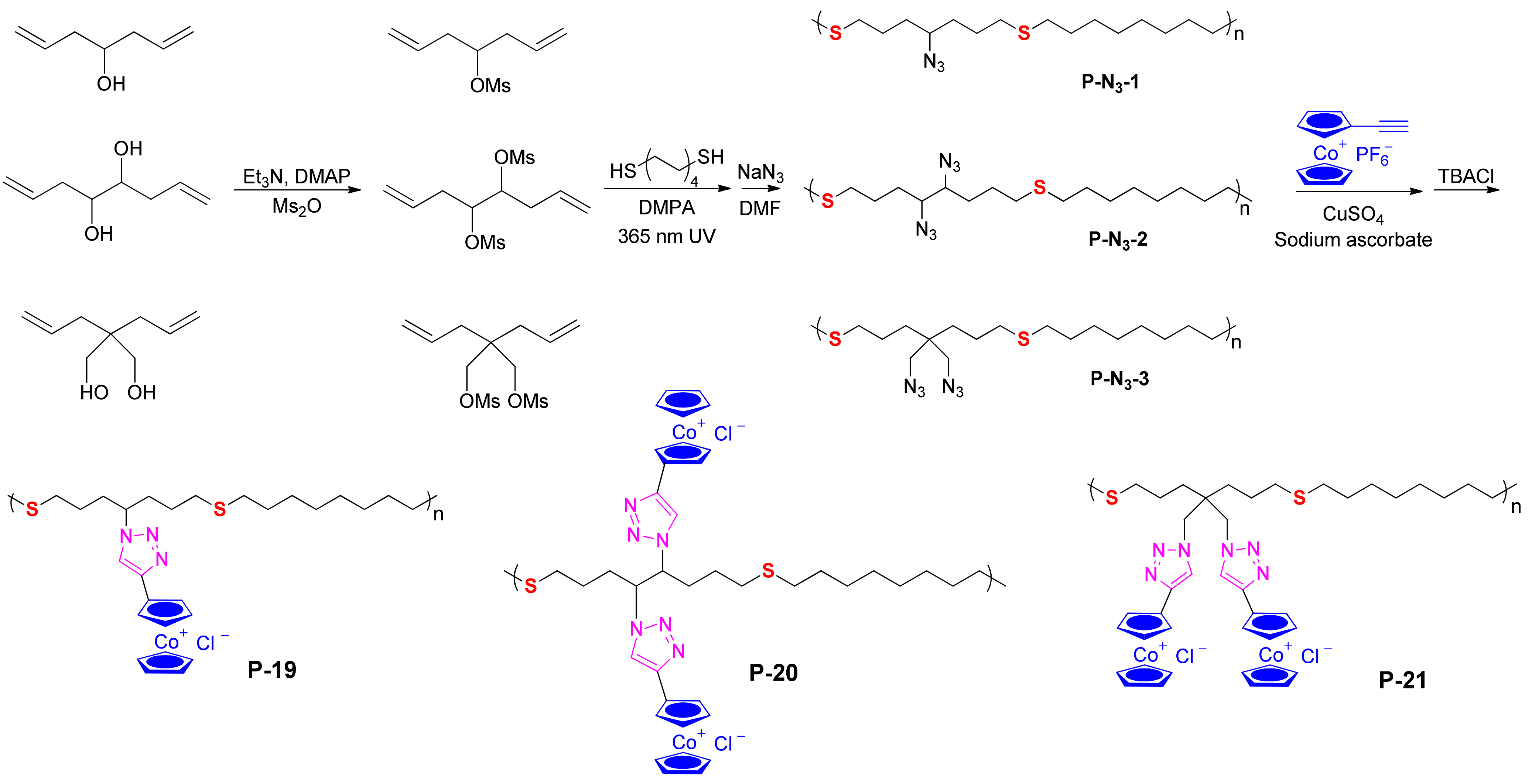
Figure 3. Synthetic procedure for cobaltocenium-containing polythioether type inhibitors: P-19/P-20/P-21 [26].
Table 2. Inhibition property of typical sulfur-containing synthetic polymeric inhibitor.
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corrosion Medium | Test Method | Highest IE (%) | Reference | ||||||||
| P-9 | 1018 carbon steel | 0.5 M H2SO4 | EIS | / | [16] | ||||||
| P-38 | N80 steel sheet | 1 M H2SO4 | PDP, EIS | 90.2 | [27] | ||||||
| P-10 | iron | ||||||||||
| P-39 | 3.5% NaCl | EIS, PDP | ST-12 type steel sheets | 96 | [21] | ||||||
| 0.1 M NaCl | PDP, EIS | / | [ | 28 | ] | P-11/P-14 | mild steel | 1 M HCl | EIS | 99 | [23] |
| P-12/P-13 | mild steel | 1 M HCl | Weight loss | P-12: 94 P-13: 87 |
[24] | ||||||
| P-15 | St37 carbon steel | 15% HCl/15% H2SO4 | EIS, PDP, linear polarization resistance, electrochemical frequency modulation | 79.5/61.1 | [19] | ||||||
| P-16/P-17/P-18 | mild steel | 1 M HCl | Weight loss | P-16: 92.3 P-18: 95.7 |
[25] | ||||||
| P-19/P-20/P-21 | mild steel | 5% HCl | weight loss, EIS, PDP | 95 | [26] | ||||||
| P-22/P-23 | cold-rolled mild steel | 3.5% NaCl | weight loss, | / | [18] |
3. Nitrogen-Containing Synthetic Polymeric Inhibitor
Nitrogen-containing synthetic polymeric inhibitor represents a major class of polymeric inhibitors. Most of the nitrogen-containing polymeric inhibitors belong to polyelectrolyte, such as poly(quaternary ammonium), polyethyleneimine, polyaniline, and so on.4. Other Type of Polymeric Inhibitors
In addition to the above well-studied systems, other kinds of polymeric inhibitors have also been investigated because modern polymer chemistry allows the introduction of hydrophilic and adsorption groups to the side chains on the basis of the main chain structure and copolymerization with other monomers (Table 3). Polyacrylic acid (PAA) is the most well-known vinyl polymer corrosion inhibitor used in the early study of anti-corrosion. Recently, polyacrylate or acrylamide copolymer corrosion inhibitors have become more popular. Lin et al. [27] prepared poly(methyl acrylate)-co-poly(acrylic acid imidazoline) (MA-ACI, P-38 in Figure 4) from methyl acrylate and acrylic imidazoline with azo diisobutyronitrile as initiator. According to the rotating hanging plate method, P-38 showed inhibition efficiency as high as 82% at a concentration of 0.10 g/L in 1 mol/L H2SO4 at 30 °C. Taghi et al. [28] prepared neodymium-poly acrylic acid complex (Nd-PAA, P-39 in Figure 4) by adding neodymium to PAA and applied them to the corrosion protection of ST-12 type in 0.1 M NaCl. Due to the anionic nature of PAA, they deposited densely and crack-free ultrafine Nd-PAA films on the steel surface.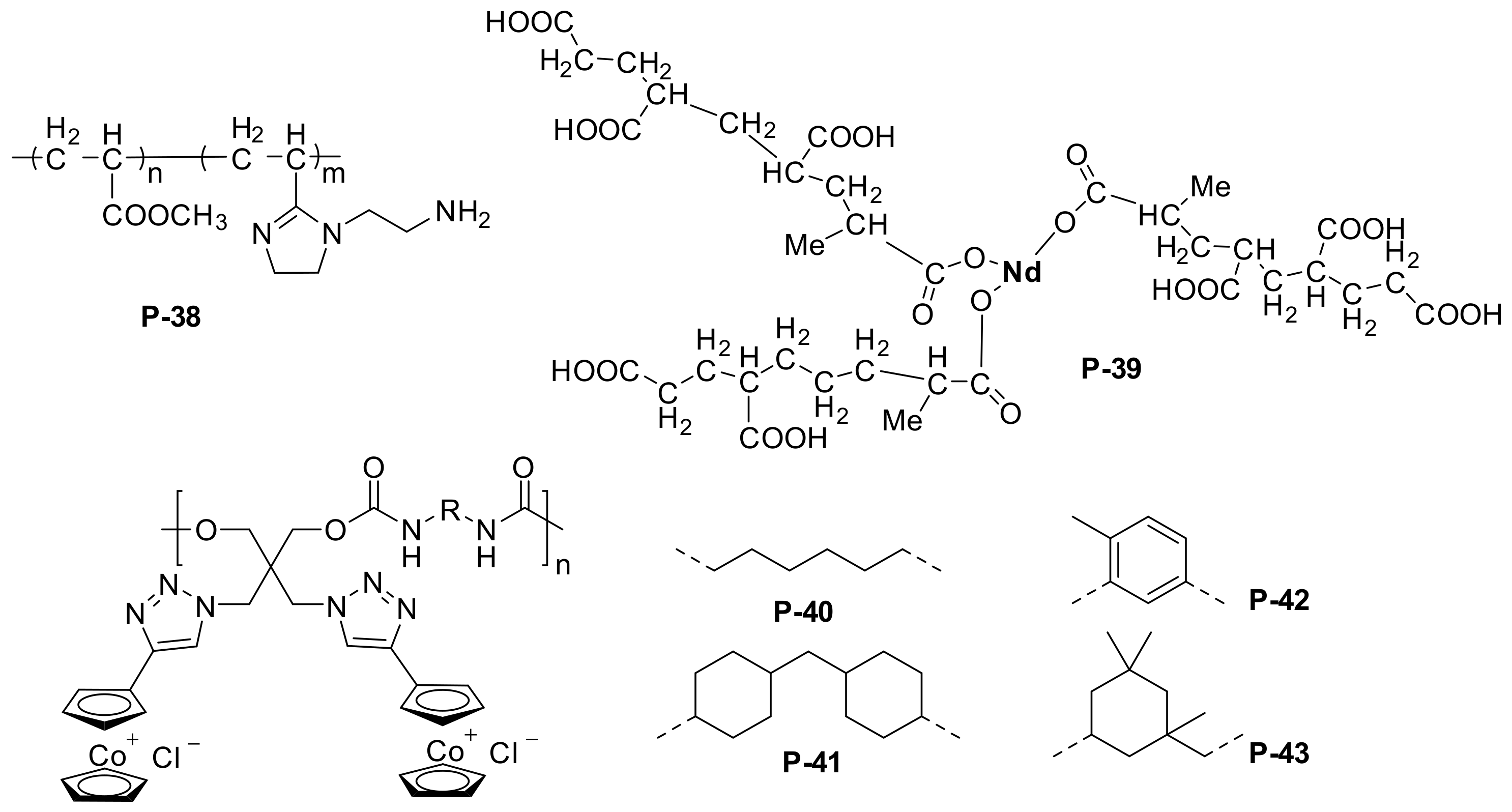
Figure 4.
Chemical structure of the poly methyl acrylate-acrylic acid imidazoline, and cobaltocenium-containing waterborne polymeric inhibitors.
Table 3. Inhibition property of other types of polymeric inhibitor.
| Inhibitor | Metal | ||||
|---|---|---|---|---|---|
| P-40/P-41/P-42/P-43 | |||||
| Mild steel | 4 M HCl | weight loss, EIS, PDP | 98 | [ | 30] |
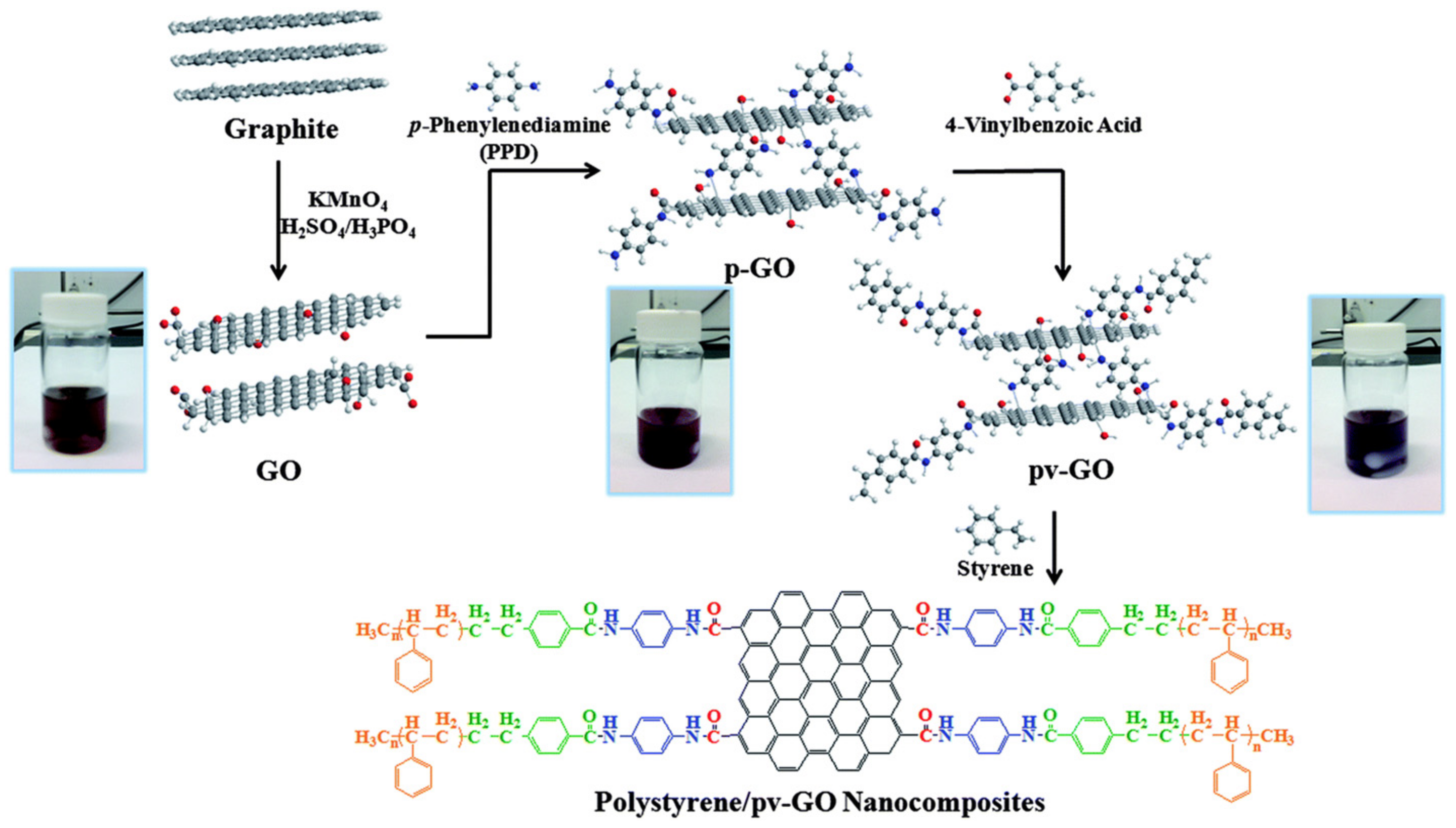
Figure 5.
Synthesis of organo-functionalized graphene oxide
[29]
. Reproduced with permission from ref.
[29]
. Copyright 2014 Royal Society of Chemistry.
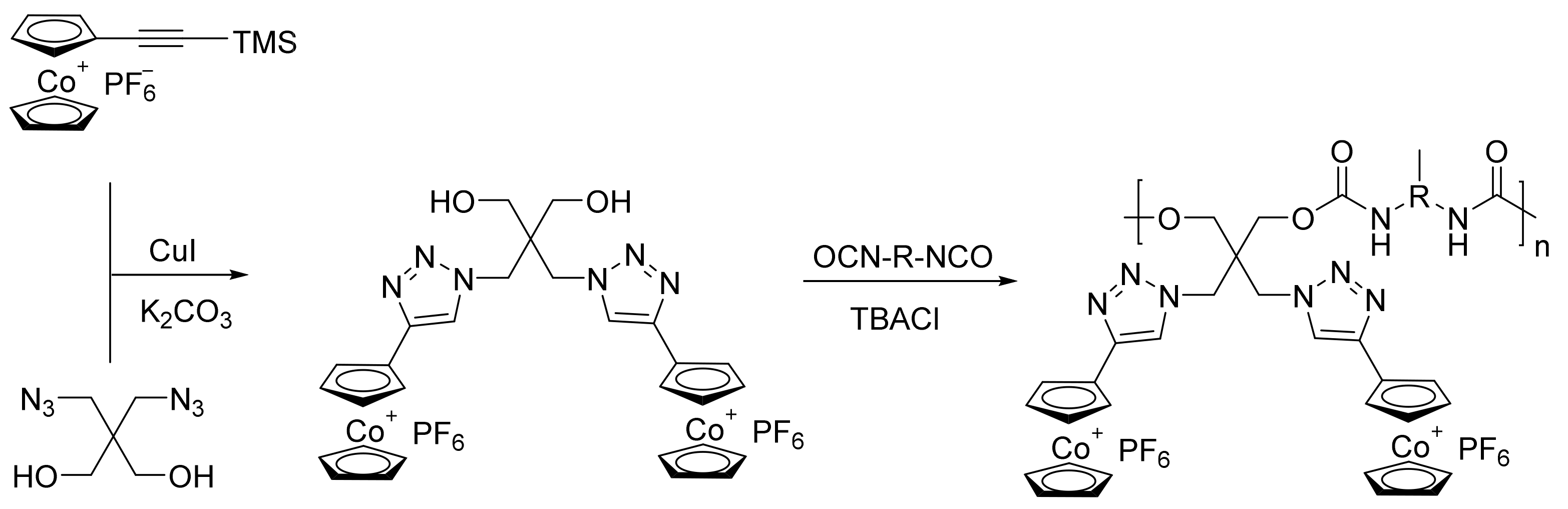
Figure 6. Synthetic procedure for cobaltocenium-containing polyurethane-type inhibitors: P-40/P-41/P-42/P-43 [30].
References
- Coquery, C.; Negrell, C.; Causse, N.; Pebere, N.; David, G. Synthesis of new high molecular weight phosphorylated chitosans for improving corrosion protection. Pure Appl. Chem. 2019, 91, 509–521.
- Solimando, X.; Kennedy, E.; David, G.; Champagne, P.; Cunningham, M.F. Phosphorus-containing polymers synthesised via nitroxide-mediated polymerisation and their grafting on chitosan by grafting to and grafting from approaches. Polym. Chem. 2020, 11, 4133–4142.
- Coquery, C.; Carosio, F.; Negrell, C.; Causse, N.; Pebere, N.; David, G. New bio-based phosphorylated chitosan/alginate protective coatings on aluminum alloy obtained by the LbL technique. Surf. Interfaces 2019, 16, 59–66.
- Ouarga, A.; Noukrati, H.; Iraola-Arregui, I.; Elaissari, A.; Barroug, A.; Ben Youcef, H. Development of anti-corrosion coating based on phosphorylated ethyl cellulose microcapsules. Prog. Org. Coat. 2020, 148, 70.
- Macarie, L.; Ilia, G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092.
- Posner, R.; Sundell, P.E.; Bergman, T.; Roose, P.; Heylen, M.; Grundmeier, G.; Keil, P. UV-Curable Polyester Acrylate Coatings: Barrier Properties and Ion Transport Kinetics Along Polymer/Metal Interfaces. J. Electrochem. Soc. 2011, 158, C185–C193.
- Millet, F.; Auvergne, R.; Caillol, S.; David, G.; Manseri, A.; Pebere, N. Improvement of corrosion protection of steel by incorporation of a new phosphonated fatty acid in a phosphorus-containing polymer coating obtained by UV curing. Prog. Org. Coat. 2014, 77, 285–291.
- Macarie, L.; Pekar, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in Aqueous Solution of Homo- and Copolymers of Vinylphosphonic Acid Derivatives Obtained by UV-Curing. Macromol. Res. 2017, 25, 214–221.
- Kousar, F.; Moratti, S.C. Synthesis of fluorinated phosphorus-containing copolymers and their immobilization and properties on stainless steel. RSC Adv. 2021, 11, 38189–38201.
- Sheffer, M.; Groysman, A.; Starosvetsky, D.; Savchenko, N.; Mandler, D. Anion embedded sol-gel films on Al for corrosion protection. Corros. Sci. 2004, 46, 2975–2985.
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Electrochemical performance of sol-gel derived phospho-silicate-methacrylate hybrid coatings. J. Electroanal. Chem. 2010, 641, 28–34.
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Synthesis and characterization of methacrylate phospho-silicate hybrid for thin film applications. Polymer 2007, 48, 7078–7086.
- Tuken, T.; Yazici, B.; Erbil, M. The use of polythiophene for mild steel protection. Prog. Org. Coat. 2004, 51, 205–212.
- Zhi, D.; Smyrl, W.H. Application of Electroactive Films in Corrosion Protection.2.Metal Hwxacyanometalate Films on TiO2/Ti Surfaces. J. Electrochem. Soc. 1991, 138, 1911–1918.
- Wang, W.L.; Lai, Y.H. Synthesis and characterization of a novel 1,4-naphthalene-based thiophene copolymers. Thin Solid Film. 2002, 417, 211–214.
- Medrano-Vaca, M.G.; Gonzalez-Rodriguez, J.G.; Nicho, M.E.; Casales, M.; Salinas-Bravo, V.M. Corrosion protection of carbon steel by thin films of poly (3-alkyl thiophenes) in 0.5 M H2SO4. Electrochim. Acta 2008, 53, 3500–3507.
- Branzoi, F.; Mihai, M.A.; Petrescu, S. Corrosion Protection Efficacy of the Electrodeposit of Poly (N-Methyl Pyrrole-Tween20/3-Methylthiophene) Coatings on Carbon Steel in Acid Medium. Coatings 2022, 12, 1062.
- Emira, H.S.A.; Abu-Ayana, Y.M.; El-Sawy, S.M. Modified amino resins for corrosion prevention in organic coatings. Pigment Resin Technol. 2013, 42, 298–308.
- McAndrew, T.P. Corrosion prevention with electrically conductive polymers. Trends Polym. Sci. 1997, 5, 7–12.
- Leon-Silva, U.; Nicho, M.E. Poly(3-octylthiophene) and polystyrene blends thermally treated as coatings for corrosion protection of stainless steel 304. J. Solid State Electrochem. 2010, 14, 1487–1497.
- Tsai, M.-C.; Yang, C.-R.; Tsai, J.-H.; Yu, Y.-H.; Huang, P.-T. Enhanced Corrosion Protection of Iron by Poly(3-hexylthiophene)/Poly (styrene-co-hydroxystyrene) Blends. Coatings 2018, 8, 383.
- Al-Muallem, H.A.; Mazumder, M.A.J.; Estaitie, M.K.; Ali, S.A. A novel cyclopolymer containing residues of essential amino acid methionine: Synthesis and application. Iran. Polym. J. 2015, 24, 541–547.
- Ali, S.A.; Saeed, M.T. Synthesis and corrosion inhibition study of some 1,6-hexanediamine-based N,N-diallyl quaternary ammonium salts and their polymers. Polymer 2001, 42, 2785–2794.
- Ali, S.A.; Goni, L.; Mazumder, M.A.J. Butler’s cyclopolymerizaton protocol in the synthesis of diallylamine salts/sulfur dioxide alternate polymers containing amino acid residues. J. Polym. Res. 2017, 24, 1022–9760.
- Haladu, S.A.; Umoren, S.A.; Ali, S.A.; Solomon, M.M.; Mohammed, A.R.I. Synthesis, characterization and electrochemical evaluation of anticorrosion property of a tetrapolymer for carbon steel in strong acid media. Chin. J. Chem. Eng. 2019, 27, 965–978.
- Liu, S.; Yan, J.; Shi, J.Q.; Li, X.Z.; Zhang, J.P.; Wang, X.Y.; Cai, N.J.; Fang, Q.H.; Zhang, Q.Y.; Yan, Y. Rational design of cobaltocenium-containing polythioether type metallo-polyelectrolytes as HCl corrosion inhibitors for mild steel. Polym. Chem. 2023, 14, 330–342.
- Lin, F.; Zhong, Y.; Zeng, T. Preparation and Performance Evaluation of Poly Methyl Acrylate-acrylic Acid Imidazoline with Corrosion Inhibition and Viscosity Reduction Effect. Oilfield Chem. 2018, 35, 144–149.
- Majd, M.T.; Shahrabi, T.; Ramezanzadeh, B.; Bahlakeh, G. Development of a high-performance corrosion protective functional nano-film based on poly acrylic acid-neodymium nitrate on mild steel surface. J. Taiwan Inst. Chem. Eng. 2019, 96, 610–626.
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550.
- Yan, J.; Li, X.; Zhang, X.; Liu, S.; Zhong, F.; Zhang, J.; Zhang, Q.; Yan, Y. Metallo-Polyelectrolyte-Based Waterborne Polyurethanes as Robust HCl Corrosion Inhibitor Mediated by Inter/intramolecular Hydrogen Bond. ACS Appl. Polym. Mater. 2022, 4, 3844–3854.
More
