Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vamsi Krishna Kukkapalli and Version 2 by Camila Xu.
A metal hydride is a compound formed between a metal and hydrogen, in which the hydrogen atoms are bonded to the metal atoms through chemical bonds. Metal hydrides have a wide range of applications as energy storage materials, catalysts, and structural materials.
- metal hydride reactor
- heat transfer
- thermal management
1. Introduction
A metal hydride is a compound formed between a metal and hydrogen, in which the hydrogen atoms are bonded to the metal atoms through chemical bonds. Metal hydrides have a wide range of applications as energy storage materials, catalysts, and structural materials. There are various types of metal hydrides, each with their own unique properties and challenges. These include interstitial, substitutional, and complex types. Interstitial hydrides are those in which the hydrogen atoms are located between the metal atoms in the crystal lattice, while substitutional hydrides are those in which the hydrogen atoms replace metal atoms in the crystal lattice. In complex hydrides, the hydrogen atoms form covalent bonds with the metal atoms, resulting in a compound with a more complex chemical structure. Some of the most well-known metal hydrides include sodium aluminum hydride (NaAlH4), magnesium hydride (MgH2), and titanium hydride (TiH2). These hydrides can store and release large amounts of hydrogen in a relatively safe and controlled manner, making them ideal for energy storage applications.
2. Metal Hydrides
One of their main characteristics is the ability to absorb and release hydrogen gas through processes known as hydriding and dehydriding, respectively [1]. This property makes them attractive for use as hydrogen storage materials, as they can store large amounts of hydrogen at relatively low pressures [2]. Metal hydrides can also be used as catalysts in chemical reactions and as structural materials due to their high strength and low density.
There are several advantages to using metal hydrides for hydrogen storage:
High capacity: Metal hydrides have a high hydrogen storage capacity, meaning that they can store large amounts of hydrogen in a relatively small volume. This makes them a compact and efficient storage option.
Safe: Metal hydrides are generally considered to be a safe storage option because they do not release hydrogen gas unless they are subjected to specific conditions, such as high temperatures or pressures. This reduces the risk of explosions or fires.
Stable: Metal hydrides are stable and do not react with other materials, making them a safe and reliable storage option.
Reusable: Metal hydrides can be used to store and release hydrogen multiple times, making them a reusable and environmentally friendly storage option.
Lightweight: Metal hydrides are typically lightweight, making them a suitable storage option for applications where weight is a concern, such as in vehicles.
Some of the applications of metal hydrides are provided in Table 1.
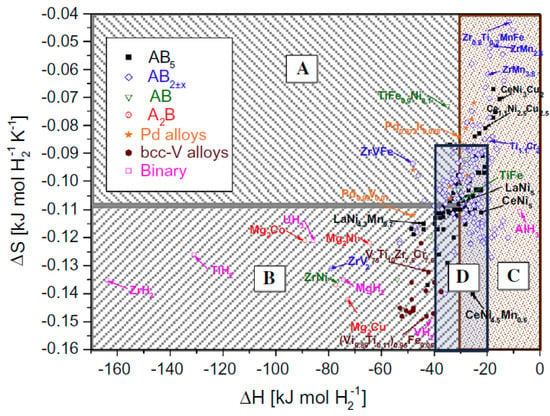
Overall, metal hydrides offer several advantages over traditional hydrogen storage methods and may be a more efficient and safe option in certain applications. In Figure 1, hydrogenation enthalpies and entropies of various hydride materials and their suitable applications are provided.
 Overall, metal hydrides have the potential to play a significant role in a variety of applications, and their properties and behavior are an active area of research in the field of materials science.
Type AB5 metal hydrides (for which LaNi5 serves as the paradigm) and type AB2 metal hydrides are the most well known for hydrogen absorption (such as Mn2Zn). The AB5 group has excellent hydrogenation ability at ambient temperature. However, its hydrogen capacity is typically in the range of 1 to 1.5 wt%. Metal hydrides based on magnesium (Mg and Mg2Ni) display unacceptably slow rates of hydrogenation and dehydrogenation even after significant activation at 673 K (400 °C) [25].
Overall, metal hydrides have the potential to play a significant role in a variety of applications, and their properties and behavior are an active area of research in the field of materials science.
Type AB5 metal hydrides (for which LaNi5 serves as the paradigm) and type AB2 metal hydrides are the most well known for hydrogen absorption (such as Mn2Zn). The AB5 group has excellent hydrogenation ability at ambient temperature. However, its hydrogen capacity is typically in the range of 1 to 1.5 wt%. Metal hydrides based on magnesium (Mg and Mg2Ni) display unacceptably slow rates of hydrogenation and dehydrogenation even after significant activation at 673 K (400 °C) [25].
Table 1.
Some applications where metal hydrides are used.
| References | Technology Area | Engineering System | Pros | Cons | Suggested Hydrides |
|---|---|---|---|---|---|
| [3]—Krane et al., 2022 | Energy Storage | Two-reactor metal hydride system |
|
|
MgH2, TiFeH2, LaNi5H6 |
| [4]—Zhang et al., 2023 | Catalysis | Ammonia synthesis |
|
|
Lanthanum hydride |
| [5]—Lv, Y. et al., 2023 | Energy storage | Magnesium hydride conversion electrode |
|
|
Magnesium hydride |
| [6]—Zhou et al., 2023 | Fuel cells | Hydrogen feeding system |
|
|
RE-based and Ti-based multicomponent metal hydrides |
| [7]—Tiwari and Sharma, 2023 | Energy storage | Metal hydride reactor and thermocline-based heat storage system |
|
|
MgH2 |
| [8]—Alok Kumar, P. Muthukumar, 2022 | Hydrogen storage and purification |
|
|
La0.9Ce0.1Ni5 | |
| [9]—Krishnamoorthy et al., 2023 | Battery modeling | Lithium–ion and nickel–metal hydride batteries |
|
Nickel-metal hydride battery | |
| [10]—Zhang et al., 2022 | Chemicals | CO2 capture |
|
|
Nickel hydride complexes |
| [11]—Brestovič, T. et al. 2022 | Metal Hydride Compressors | Heat pump-based compression system |
|
|
LaNi5 |
| [12]—Massaro et al., 2023 | On-board hydrogen storage technologies | Fuel cell systems for aircraft electrification |
|
|
|
| [13]—Nguyen and Shabani, 2022 | Metal hydride hydrogen storage | Standalone solar hydrogen systems |
|
|
|
| [14]—Eadi et al., 2023 | Hydrogen gas sensing | Pd-Ni alloy thin films |
|
|
|
| [15]—Kumar et al., 2022 | Metal hydride-based hydrogen storage | Standalone microgrids |
|
|
MgH2, TiFeH2, LaNi4.8Al0.2H11.3 |
| [16]—Tian et al., 2022 | Hybrid rocket propulsion | Solid-fuel additives |
|
|
|
| [17]—Lee et al., 2022 | Hydrogen storage | Magnesium hydrogen tank |
|
|
MgH2 |
| [18]—Sezgin et al., 2022 | Hydrogen energy systems | Underwater applications |
|
|
TiFe, LaNi5, AB2 (MmNi3.6Co0.7Mn0.4Al0.3), MgH2, LiBH4 |
| [19]—Kailiang Ren, Jiajia Miao, et al., 2022 | Electrochemical energy storage | Batteries |
|
|
NiMH |
| [20]—Dinesh Dashbabu, E. Anil Kumar, I.P. Jain, 2022 | Hydrogen compression | Metal hydride hydrogen compressor |
|
|
LaNi5H6-xAlx |
| [21]—Sayantan Jana et al., 2022 | Heating and cooling | Embedded cooling tube type metal hydride reactor |
|
|
Mg2NiH4, Top of Form |
| bottom of form | |||||
| [22]—Singh et al., 2022 | Renewable energy | Reversible SOFC, hydrogen storage, rankine cycle, absorption refrigeration |
|
|
LaNi4.8Al0.2, MgH2, Ni-MH alloy, CaH2 |
| [23]—H. Chang, Y.B. Tao, H. Ye, 2023 | Hydrogen and thermal storage | Sandwich reaction bed filled with metal hydride and thermochemical material |
|
|

Figure 1. Hydrogenation enthalpies and entropies of various hydride materials and their suitable applications: (A) heat pumps, (B) heat storage, (C) hydrogen storage, (D) hydrogen compression [24].
References
- Huot, J. Metal Hydrides. In Handbook of Hydrogen Storage; Hirscher, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 81–116. ISBN 9783527322732.
- Sun, D.-W.; Deng, S.-J. A Theoretical Model Predicting the Effective Thermal Conductivity in Powdered Metal Hydride Beds. Int. J. Hydrogen Energy 1990, 15, 331–336.
- Krane, P.; Nash, A.L.; Ziviani, D.; Braun, J.E.; Marconnet, A.M.; Jain, N. Dynamic Modeling and Control of a Two-Reactor Metal Hydride Energy Storage System. Appl. Energy 2022, 325, 119836.
- Zhang, X.; Liu, L.; Wang, J.; Ju, X.; Si, R.; Feng, J.; Guo, J.; Chen, P. The Role of Lanthanum Hydride Species in La2O3 Supported Ru Cluster Catalyst for Ammonia Synthesis. J. Catal. 2023, 417, 382–395.
- Lv, Y.; Zhang, X.; Chen, W.; Ju, S.; Liu, Z.; Xia, G.; Ichikawa, T.; Zhang, T.; Yu, X. Ion Diffusion, and Hysteresis of Magnesium Hydride Conversion Electrode Materials. J. Mater. Sci. Technol. 2023, 155, 47–53.
- Zhou, P.; Cao, Z.; Xiao, X.; Zhan, L.; He, J.; Zhao, Y.; Wang, L.; Yan, M.; Li, Z.; Chen, L. Development of RE-Based and Ti-Based Multicomponent Metal Hydrides with Comprehensive Properties Comparison for Fuel Cell Hydrogen Feeding System. Mater. Today Energy 2023, 33, 101258.
- Tiwari, S.; Sharma, P. Integration of Metal Hydride Reactor with Thermocline Based Heat Storage System. J. Energy Storage 2023, 59, 106506.
- Kumar, A.; Muthukumar, P. Experimental Investigation on the Poisoning Characteristics of Methane as Impurity in La0.9Ce0.1Ni5 Based Hydrogen Storage and Purification System. Energy 2022, 259, 124888.
- Krishnamoorthy, U.; Gandhi Ayyavu, P.; Panchal, H.; Shanmugam, D.; Balasubramani, S.; Al-rubaie, A.J.; Al-khaykan, A.; Oza, A.D.; Hembrom, S.; Patel, T.; et al. Efficient Battery Models for Performance Studies-Lithium Ion and Nickel Metal Hydride Battery. Batteries 2023, 9, 52.
- Zhang, M.; Liang, X.; Wang, Y.; Yang, H.; Liang, G. Insights into the Capture of CO2 by Nickel Hydride Complexes. Catalysts 2022, 12, 790.
- Brestovič, T.; Jasminská, N.; Lázár, M. Measurements of Operating Parameters of a Metal Hydride Compressor with a Heat Pump. Appl. Sci. 2022, 12, 3302.
- Massaro, M.C.; Biga, R.; Kolisnichenko, A.; Marocco, P.; Monteverde, A.H.A.; Santarelli, M. Potential and Technical Challenges of On-Board Hydrogen Storage Technologies Coupled with Fuel Cell Systems for Aircraft Electrification. J. Power Sources 2023, 555, 232397.
- Nguyen, H.Q.; Shabani, B. Thermal Management of Metal Hydride Hydrogen Storage Using Phase Change Materials for Standalone Solar Hydrogen Systems: An Energy/Exergy Investigation. Int. J. Hydrogen Energy 2022, 47, 1735–1751.
- Eadi, S.B.; Oh, J.S.; Kim, C.; Sim, G.; Kim, K.; Kim, H.Y.; Kim, J.J.; Do, H.R.; Chu, S.; Jung, S.H.; et al. Improved Hydrogen Gas Sensing Performance of Pd–Ni Alloy Thin Films. Int. J. Hydrogen Energy 2023, 48, 12534–12539.
- Kumar, S.; Sharma, R.; Srinivasa Murthy, S.; Dutta, P.; He, W.; Wang, J. Thermal Analysis and Optimization of Stand-Alone Microgrids with Metal Hydride Based Hydrogen Storage. Sustain. Energy Technol. Assess. 2022, 52, 102043.
- Tian, H.; Wang, Z.; Guo, Z.; Yu, R.; Cai, G.; Zhang, Y. Effect of Metal and Metalloid Solid-Fuel Additives on Performance and Nozzle Ablation in a Hydroxy-Terminated Polybutadiene Based Hybrid Rocket Motor. Aerosp. Sci. Technol. 2022, 123, 107493.
- Lee, C.-Y.; Shen, C.-C.; Chiu, C.-W.; Hsieh, H.-T. Real-Time Micro-Monitoring of Surface Temperature and Strain of Magnesium Hydrogen Tank through Self-Made Two-In-One Flexible High-Temperature Micro-Sensor. Micromachines 2022, 13, 1370.
- Sezgin, B.; Devrim, Y.; Ozturk, T.; Eroglu, I. Hydrogen Energy Systems for Underwater Applications. Int. J. Hydrogen Energy 2022, 47, 19780–19796.
- Ren, K.; Miao, J.; Shen, W.; Su, H.; Pan, Y.; Zhao, J.; Pan, X.; Li, Y.; Fu, Y.; Zhang, L.; et al. High Temperature Electrochemical Discharge Performance of LaFeO3 Coated with C/Ni as Anode Material for NiMH Batteries. Prog. Nat. Sci. Mater. Int. 2022, 32, 684–692.
- Dashbabu, D.; Kumar, E.A.; Jain, I.P. Thermodynamic Analysis of a Metal Hydride Hydrogen Compressor with Aluminium Substituted LaNi5 Hydrides. Int. J. Hydrogen Energy 2022.
- Jana, S.; Raju, N.N.; Muthukumar, P. Performance Tests on Embedded Cooling Tube Type Metal Hydride Reactor for Heating and Cooling Applications. Therm. Sci. Eng. Prog. 2022, 33, 101349.
- Raj Singh, U.; Sai Kaushik, A.; Sekhar Bhogilla, S. A Novel Renewable Energy Storage System Based on Reversible SOFC, Hydrogen Storage, Rankine Cycle and Absorption Refrigeration System. Sustain. Energy Technol. Assess. 2022, 51, 101978.
- Chang, H.; Tao, Y.B.; Ye, H. Numerical Study on Hydrogen and Thermal Storage Performance of a Sandwich Reaction Bed Filled with Metal Hydride and Thermochemical Material. Int. J. Hydrogen Energy 2023.
- Lototskyy, M.; Satya Sekhar, B.; Muthukumar, P.; Linkov, V.; Pollet, B.G. Niche Applications of Metal Hydrides and Related Thermal Management Issues. J. Alloys Compd. 2015, 645, S117–S122.
- Sandrock, G. Hydride Storage. In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: Chichester, UK, 2010.
More
