Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Dharmendra Kumar Yadav.
Triple-negative breast cancer (TNBC) is a more aggressive type of breast cancer due to its heterogeneity and complex molecular mechanisms. TNBC has a high risk for metastasis, and it is difficult to manage clinical conditions of the patients. Long non-coding RNAs (lncRNAs) have emerged as a novel target to treat the multistep process of TNBC. LncRNAs regulate epigenetic expression levels, cell proliferation and apoptosis, and tumour invasiveness and metastasis. Thus, lncRNA-based early diagnosis and treatment options could be helpful, especially for patients with severe TNBC.
- triple-negative breast cancer
- lncRNA
- diagnosis
- targeted drug development and resistance
1. LncRNAs
lncRNAs are actively involved in gene expression, epigenetic deregulation, chromatin remodelling, DNA methylation, translation of oncogenic gene targets, and biogenesis (Figure 1). They are transcribed by RNA polymerase II, after which most transcripts are spliced, and are mainly found in the nucleus and chromatin, being expressed in cells and tissues in a specific manner [6,9,17][1][2][3]. Transcriptional regulation and various molecular processes in the cytoplasm are controlled by lncRNAs; various circulating lncRNAs are transmitted via exosomes and bind to various transcription factors, chromatin-regulated complexes, RNA-binding proteins, nascent RNA transcripts, and chromatin [17][3]. The normal expression of lncRNAs and the effect of their expression changes on tumour behaviour depends on the canonical function of the mRNA target genes (Figure 2). lncRNAs can bind to the active site of proteins and regulate molecular processes at the post-transcriptional level. They are involved in functional biological processes at the cellular or physiological levels. RNA-induced silencing complexes (RISCs) are formed with the help of lysine-specific demethylase 5B (KDM5B, also known as histone demethylase JARID1B), trimethylation of lysine 4 on the histone H3 protein subunit (H3K4me3), monomethylation of lysine 4 on the histone H3 protein subunit (H3K4me1), hsa-miR-448 (also known as miRNA448), breast cancer 1/2 (BRCA1/2), retinoblastoma protein (pRB), caveolin-1 (CAV-1), Homeobox protein Hox-A5 (HOXA5), Stratifin (SFN), methyl groups (CH3), and Ras homolog gene family, member A (RhoA) (Figure 1 and Figure 3) [18][4]. In 2019, it was found that the lncRNA MIR100HG regulates proliferation in TNBC and the expression of the p27 gene after formation of an RNA–DNA triplex at the promoter [19][5]. Moreover, MIR100HG silencing leads to reduced transcription and translation of p27 [19,20][5][6]. Three triplex-forming oligonucleotides (TFOs) have been observed on the lncRNA of p27, which binds to the triplex-targeting ability (TTA) site at the 5’UTR; this event has been observed in TNBC cell lysates [21][7]. The binding of TFO1 and TTA is a unique mechanism by which MIR100HG regulates the transcription factors at the promoter region of p27 [21,22][7][8]. Plasmacytoma variant translocation 1 (PVT1) is another type of lncRNA that is transcribed by a gene situated at the 8q24 chromosomal region and plays and important role in TNBC development. It contains 12 exons that when spliced generate lncRNAs [23][9]. PVT1 binds to Krüppel-like factor 5 (KLF5) and generates a BAP1 deubiquitinase that induces TNBC via beta-catenin upregulation. Furthermore, the PVT1 promoter also acts as a regulator of the expression of the MYC proto-oncogene and BHLH transcription factor (c-MYC) [24][10]. These findings show that lncRNAs also mediate regulation at the transcriptional level.
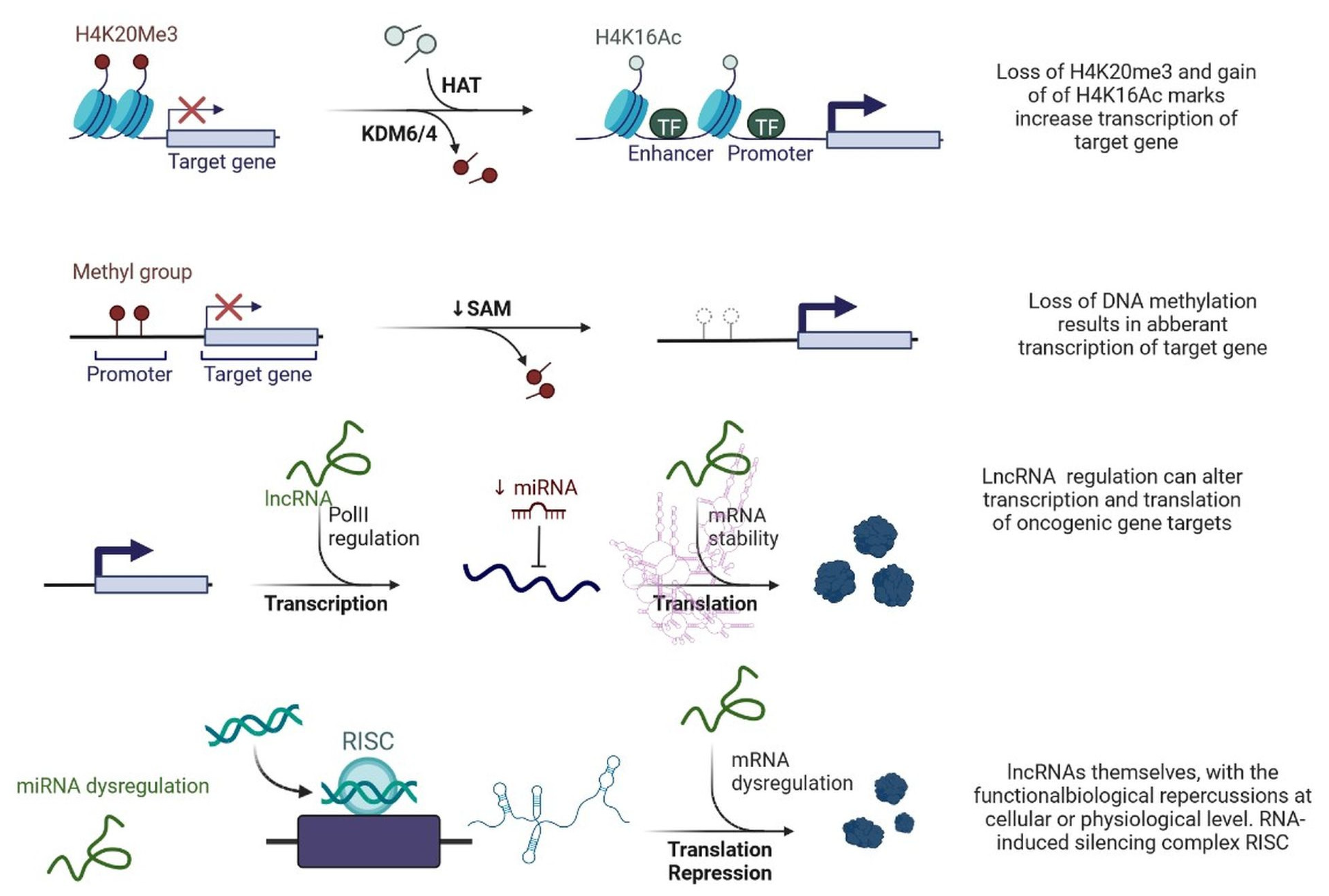
Figure 1. Epigenetic deregulation in cancer including chromatin remodelling, DNA methylation, and non-coding RNA regulation that alters transcription and translation of oncogenic gene targets.
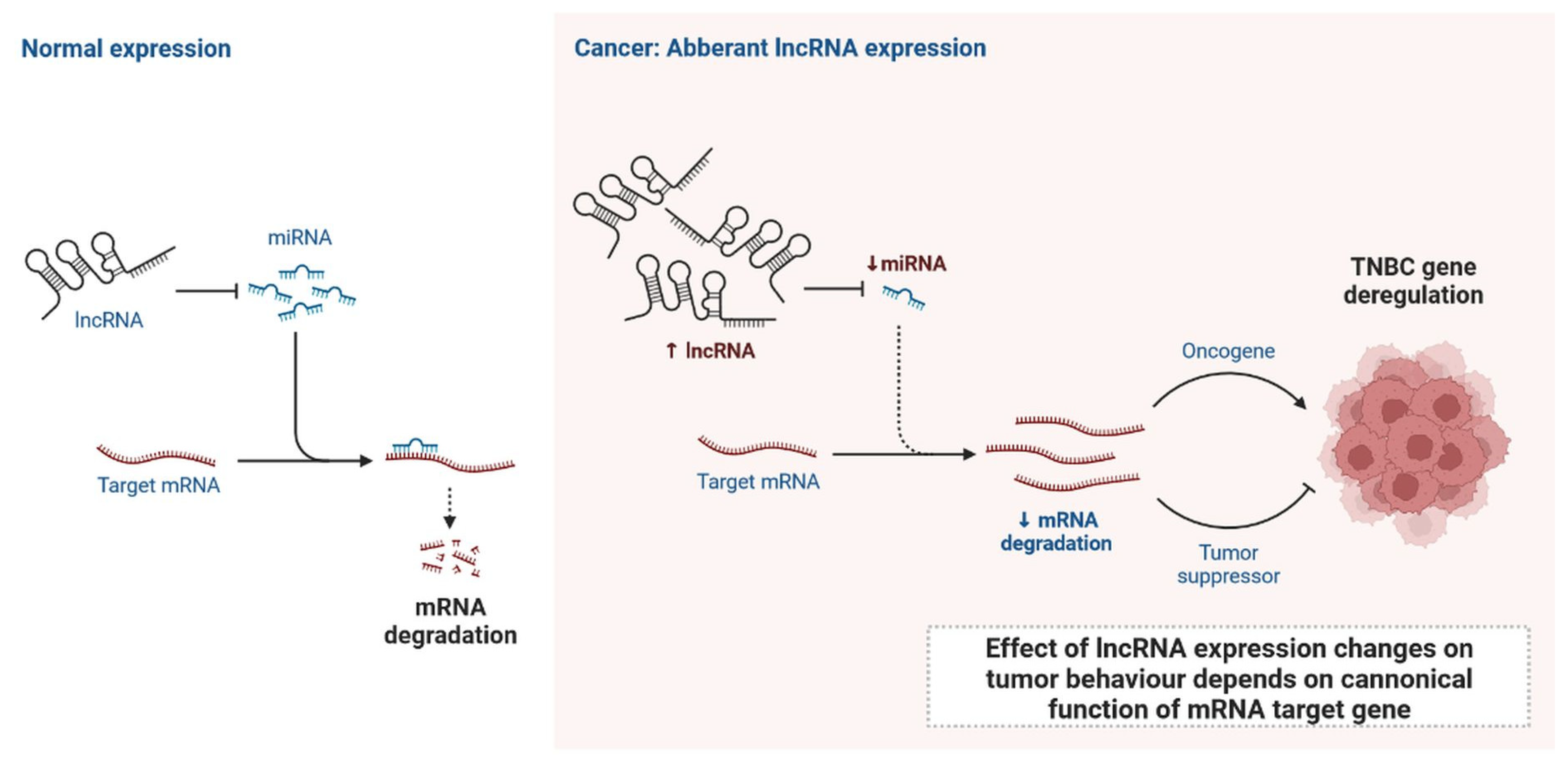
Figure 2. Normal expression of lncRNA and effect of lncRNA expression changes on tumour behaviour depends on canonical function of mRNA target gene.
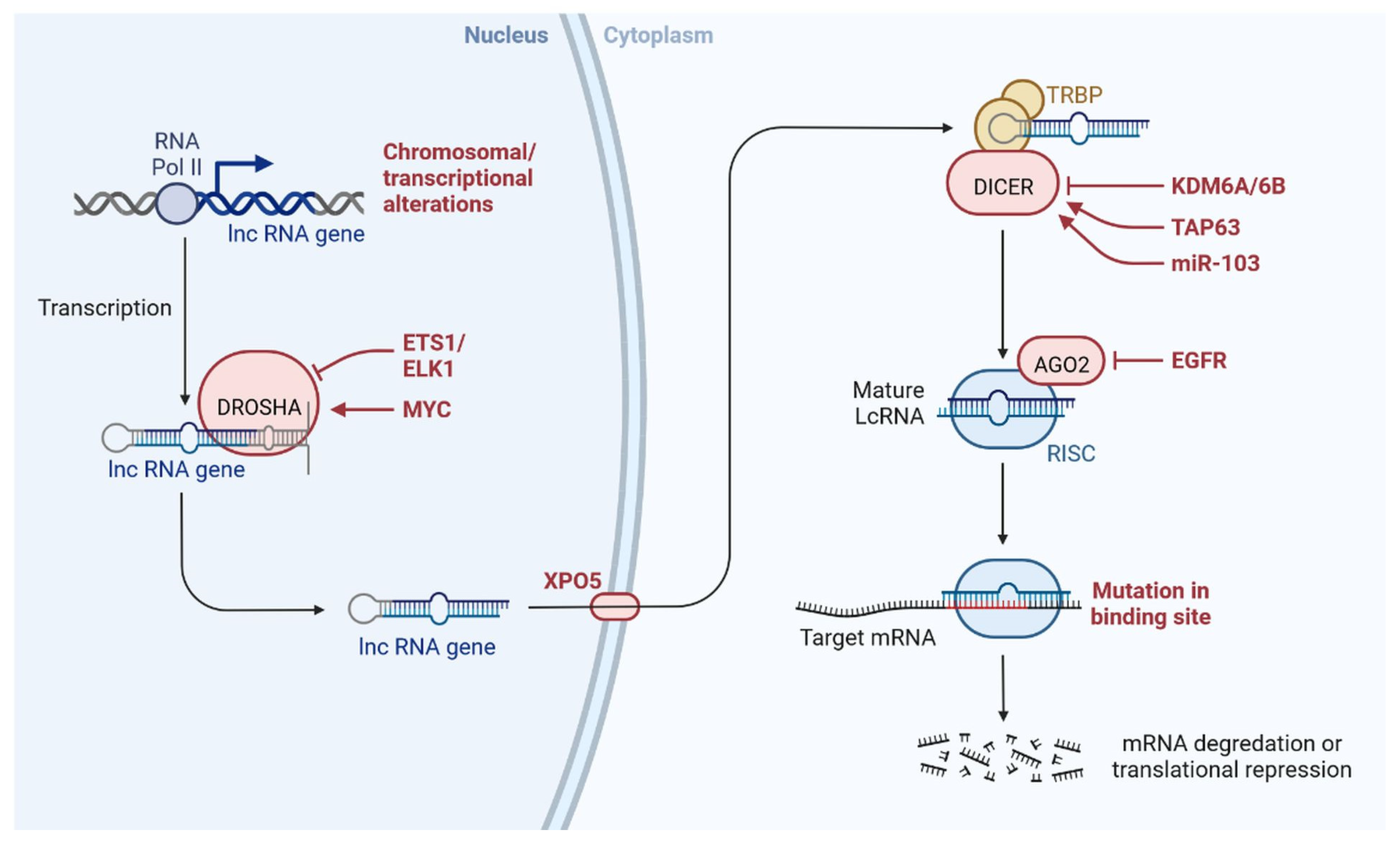
Figure 3. lncRNAs are involved with the functional repercussions at the cellular and physiological level. RNA-induced silencing complex (RISC): KDM5B (lysine-specific demethylase 5B also known as histone demethylase JARID1B), H3K4me3 (trimethylation of lysine 4 on the histone H3 protein subunit), H3K4me1 (monomethylation of lysine 4 on the histone H3 protein subunit), hsa-miR-448 (also known miRNA448), BRCA1/2 (breast cancer 1/2), pRB (retinoblastoma protein), CAV 1 (caveolin 1), HOXA5 (Homeobox protein Hox-A5), SFN (Stratifin), CH3 (methyl group), and RhoA (Ras homolog gene family, member A).
2. Clinical Updates on lncRNAs in TNBC
Recently, lncRNA expression in patients with TNBC was investigated; 1034 lncRNAs were identified using NGS technologies and microarrays, out of which, 537 lncRNAs regulate 451 protein-coding genes [14][11]. These genes are also detected in TNBC cells and are involved in cell signalling pathways such as the MAPK and PI3K-Akt pathways, which may lead to heterogeneity [14,24][10][11]. lncRNAs also act as miRNAs, binding to miRNA-targeted mRNAs and dysregulated miRNAs [25][12]. This crosstalk forms a complex post-transcriptional regulatory network including mRNAs and lncRNAs that is called the competing endogenous RNA (ceRNA) network [26][13]. ceRNA-mediated regulatory mechanisms constitute an important pathway in lncRNA-modulated post-transcriptional regulation in TNBC [27][14]. A microarray-based ceRNA network analysis revealed that 4852 lncRNAs are related to the diagnosis and treatment outcome of TNBC [28][15]. Another study using the TCGA database found that 150 lncRNAs are expressed at the tissue level and 823 in serum and these lncRNAs could act as prognostic factors in TNBC [29][16]. Furthermore, the study found that the lncRNA OSTN-AS1 is a novel immune-related prognostic marker [29][16]. An integrated ceRNA network involving three miRNAs (CHRDL1, FCGR1A, and RSAD2) and two lncRNAs (HIF1A-AS2 and AK124454) was developed using microarray analysis [30][17]. These findings demonstrate that lncRNAs play major roles in the regulation of cell signalling, genetic heterogeneity, TNBC development, and pathological features (Figure 4) shown in Table 1.
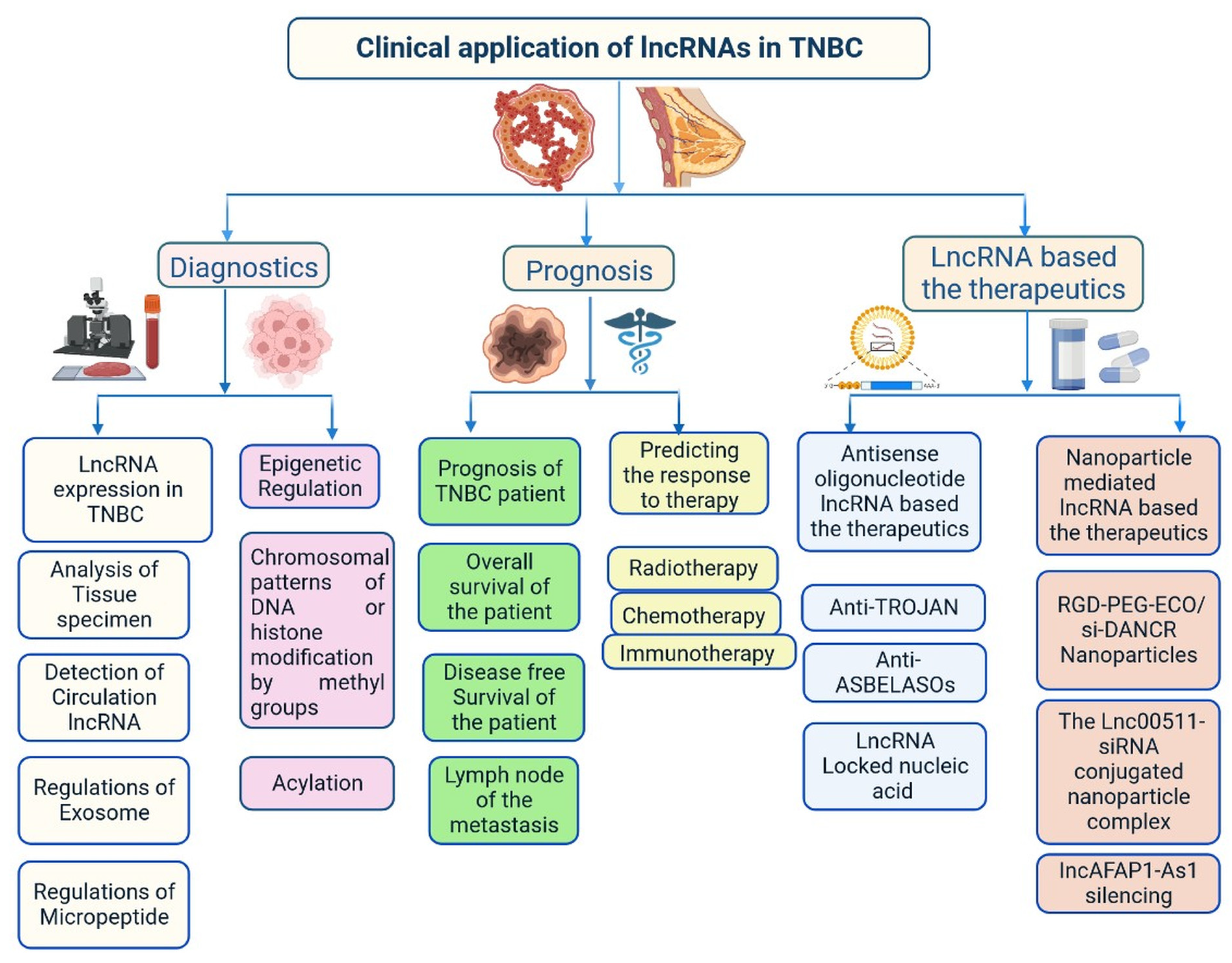
Figure 4.
Clinical importance of lncRNA in triple-negative breast cancer.
Table 1.
Important lncRNAs associated with triple-negative breast cancer.
| S. N. | lncRNAs | Regulation of Expression | Clinical Importance | Potential Targets | Reference |
|---|---|---|---|---|---|
| 1 | HOTAIR | Upregulation | Increase cell invasion and migration | LEF1/TCF4 | [31][18] |
| 2 | DRHC | Downregulation | Reduce cell proliferation | HOTAIR | [32][19] |
| 3 | LINC01133 | Upregulation | Promote phenotypic features like cell stem cells (CSCs) | KLF4 | [33][20] |
| 4 | LINC01096 | Upregulation | Encourage cell invasion | miR-3130-3p | [34][21] |
| 5 | HEIH | Upregulation | Increase cell proliferation and prevent cell death | miR-4458/SOCS1 | [35][22] |
| 6 | ARNILA | Downregulation | Invasion and metastasis | miR-204/SOX4 | [36][23] |
| 7 | LINC02095 | Upregulation | Promote cell proliferation | SOX9 | [37][24] |
| 8 | WT1-AS | Downregulation | Inhibit cell migration and invasion | TGF-β1 | [38][25] |
| 9 | GAS5 | Downregulation | Promote cell apoptosis | miR-378a-5p/SUFU | [39][26] |
| 10 | CCAT1 | Upregulation | Encourage cell division | miR-218/ZFX | [40][27] |
| 11 | ASRPS | Downregulation | Inhibit angiogenesis and cell proliferation | STAT3 | [41][28] |
| 12 | AND2-AS1 | Downregulation | Inhibit angiogenesis inhibit cell division | RUNX2 | [42][29] |
| 13 | POU3F3 | Upregulation | Promote cell proliferation and inhibit cell apoptosis | Caspase-9 | [43][30] |
| 14 | NEF | Downregulation | Inhibit cell migration and invasion | miR-155 | [44][31] |
| 15 | ZEB2-AS1 | Upregulation | Promote cell proliferation, metastasis, and EMT | ZEB2 | [45][32] |
| 16 | LINC0009 | Upregulation | Increase cell proliferation and invasion | miR-383-5p/RBM3 | [46][33] |
| 17 | ANRIL | Upregulation | Increase cell proliferation and apoptosis | miR-448/KDM5B | [47][34] |
| 18 | SNHG12 | Upregulation | Induce cell proliferation, migration, and apoptosis | MMP13 | [48][35] |
| 19 | LUCAT1 | Upregulation | Encourage cell division, movement, and invasion | miR-5702 | [49][36] |
| 20 | PCAT6 | Upregulation | Radiotherapy resistance | miR-185-5p/TPD52 | [50][37] |
| 22 | HULC | Upregulation | Promote metastasis | MMP-2, MMP-9 | [51][38] |
| 23 | PAPAS | Upregulation | Induce cell migration and invasion | miR-34a | [52][39] |
| 24 | HCP5 | Upregulation | Increase cell proliferation; reduce cell apoptosis | miR-219a-5p/BIRC3 | [53][40] |
| 25 | NRAD1 | Upregulation | Stimulate cell proliferation and CSC-like phenotypic traits | miR-219a-5p/BIRC3 | [54][41] |
| 26 | SNAR | Upregulation | Stimulate cell division | [55][42] | |
| 27 | AWPPH | Upregulation | Activate cell proliferation | miR-21; FZD7 | [56][43] |
| 28 | sONE | Downregulation | Prevent cell proliferation | TP53/c-Myc | [57][44] |
| 29 | DANCR | Upregulation | Promote cell proliferation and invasion | miR-216a-5p | [58][45] |
| 30 | LINK-A | Upregulation | Increase resistance to immunotherapy, AKT inhibitors, and glycolysis reprogramming | PI3K/GPCR | [59][46] |
| 31 | MIR503HG | Downregulation | Reduce cell migration and invasion | miR-103/OLFM4 | [60][47] |
| 32 | NEAT1 | Upregulation | Increase cell apoptosis | [61][48] | |
| 33 | PTCSC3 | Downregulation | Prevent cell proliferation | H19 | [62][49] |
| 34 | NRON | Downregulation | Inhibit cell proliferation | snaR | [63][50] |
| 35 | TROJAN | Upregulation | Promote cell proliferation and invasion | ZMYND8 | [64][51] |
| 36 | NAMPT-AS | Upregulation | Increase cell metastasis | miR-548b-3p/NAMPT | [14][11] |
| 37 | MANCR | Upregulation | Promote cell proliferation; inhibit DNA damage | [65][52] | |
| 38 | RMST | Downregulation | Prevent cell proliferation | [66][53] | |
| 39 | SK AI1BC | Upregulation | Increase cell migration and invasion | K AI1 | [67][54] |
| 40 | ROR | Upregulation | Promote cell invasion and metastasis | miR-145/ARF6 | [68][55] |
| 41 | AIRN | Downregulation | Inhibit cell migration and invasion | Wnt/β-catenin/mTOR/PI3K | [69][56] |
| 42 | LINC-ZNF469-3 | Upregulation | Promote cell invasion | miR-574-5p/ZEB1 | [70][57] |
| 43 | PDCD4-AS1 | Downregulation | Inhibit cell proliferation and migration | PDCD4 | [71][58] |
| 44 | HOST2 | Downregulation | Inhibit cell proliferation | et-7 b/CDK6 | [72][59] |
| 45 | BORG | Upregulation | Promote doxorubicin resistance | RPA1 | [73][60] |
| 46 | PVT1 | Upregulation | Promote cell proliferation and migration, and EMT | p21, KLF5/β-catenin | [24][10] |
| 47 | H19 | Upregulation | Promote paclitaxel resistance and CSC-like phenotypic traits | Akt | [62][49] |
| 48 | TP73-AS1 | Downregulation | Promote cell vasculogenic mimicry | miR-490-3p/TWIST1 | [74][61] |
| 49 | TUG1 | Downregulation | Enhance cisplatin sensitivity | miR-197/NLK | [75][62] |
| 50 | MIR100HG | Upregulation | Promote cell proliferation | p27 | [76][63] |
| 51 | LINC01638 | Upregulation | Promote cell proliferation | c-Myc | [77][64] |
2.1. Importance of lncRNAs in Tumour Invasiveness and Metastasis
Tumour invasion and metastasis explain the severity and mortality rate in patients with TNBC (Figure 64) [78,79][65][66]. GAS5 overexpression induces the expression of miR-196a-5p, which activates the FOXO1/PI3K/Akt signalling pathway [80][67]. TROJAN is a drug that reduces the metastasis burden. Degradation of TROJAN is regulated by ZMYND8, and the ubiquitin–proteasome pathway is involved in this process [81][68]. CCAT1 activates the migration of TNBC cells via miR-218/ZFX signalling [40][27]. Various ncRNAs are involved in cell migration and invasion via specific regulatory pathways, including MIR503HG through the miR-103/OLFM4 axis [60][47], CCAT1 through the dysregulation of the miR-218/ZFX axis [40][27], AFAP1-AS1 through the activation of Wnt/β-catenin signalling [82][69], miR-34a through the activation of EMT-associated signalling pathways [83][70], PAPAS through miR-34a.83 downregulation [52][39], sONE through sONE/NOS3/NO signalling activation [53][40], LINC-ZNF469-3 by activating the miR-574-5p/ZEB1 axis [71[58][65],78], ZEB2 through the activation of PI3K/Akt/GSK3β/ZEB2 signalling [45][32], PVT1 by regulating p21 and KLF5/β-catenin signalling [24][10], ARNILA by mimicking ceRNA for miR-204, AIRN by downregulating Wnt/β-catenin/mTOR/PI3K signalling [36][23], RMST by downregulating Wnt/β-catenin/mTOR/PI3K signalling [67][54], and MALAT1 by upregulating miR-129-5p and miR-1/Slug expression [84][71]. Furthermore, miR-448 and some other lncRNAs play very important roles in invasion and metastasis, including SKAI1BC, HULC, HOTAIR, SNHG12, SNAR, WT1-AS, LINC01096, DANCR, NEF, HIF1A-AS2, LncKLHDC7B, and ROR [30,31,32,38,48,55,58,59,60,61,62,63,64,65,66,67,68,69,85,86][17][18][19][25][35][42][45][46][47][48][49][50][51][52][53][54][55][56][72][73].
2.2. Importance of lncRNAs in Clinical Diagnosis
Several studies have found that lncRNAs are involved in the regulation of various transcription factors, epigenetic changes, chromatin remodelling, DNA methylation patterns, alternative splicing, post-translational modifications, and interaction with small peptides. All these events have great importance in the early diagnosis and treatment of patients with TNBC [14,86][11][73]. lncRNA expression levels in the blood and tissues of patients with TNBC at different stages has been investigated [14][11]. Based on reverse transcription quantitative PCR analysis data, the lncRNAs HIF1A-AS2, UCA1, and ANRIL can be used for TNBC detection, with areas under the curve in the range of 0.827–0.840, and a diagnostic accuracy of 0.962 for ANRIL [87][74]. ANRIL, SOX2OT, and ANRASSF1 are used to differentiate between healthy and TNBC cells. TINCR expression is used to differentiate various histological subtypes of BC, as it is highly expressed in TNBC cells [88][75]. UCA1 is associated with TNBC, acting as a specific marker for TNBC diagnosis. EZH2 is highly expressed in TNBC tissues and prevents apoptosis by activating the miR-4458/SOCS1 axis [89][76]. LINC00299 expression is increased in TNBC. Several lncRNAs bind to mRNAs, protecting them and increasing their stability. The oncogenic transcription factor SOX9 is activated by LINC02095 [90][77]. DANCR interacts with RXRA and activates PI3K/Akt signalling in TNBC [58][45]. LINC00152 enhances NEDD4-1-facilitated ubiquitination and dysregulation of PTEN protein in TNBC [91][78]. Cell cycle arrest at the G1 phase is induced by MIR100HG, with p27 binding to RNA–DNA; p27 is a cyclin-dependent kinase (CDK) inhibitor. Cell cycle arrest at the G0/G1 phase is induced by LINC00339 and RMST in TNBC through the miR-377-3p/HOXC6 signalling pathway [19,20,77,92][5][6][64][79]. GAS5 is actively involved in the inhibition of TNBC cells through its action on miR-196a-5p and miR-378a-5p/SUFU signalling [93][80]. Further understanding of the roles of all these lncRNAs in TNBC is needed to improve early diagnosis and clinical management of patients. Various genes are targeted by ncRNAs, including LARP7, CDKN1A, KLF2, TIA1, DDX3X, CDK, and QKI [94,95,96,97,98][81][82][83][84][85]. An analysis of the TCGA database showed that 1097 lncRNAs are expressed in BC, with 1510 differentially expressed lncRNAs in TNBC cells, 35 plasma lncRNAs in TNBC, and 672 in non-TNBC cells [14][11]. Some lncRNAs are directly linked to prognosis in TNBC, including FOXCUT, LINC00299, AP000924.1, AC091043.1, AL354793.1, AC010343.3, and FGF10-AS1 [14][11]. Plasma-specific lncRNAs are also used for diagnosis of TNBC, such as UCA1, ANRIL, and HIF1A-AS2 [30][17]. lncRNAs associated with lymph node metastasis, such as LINC000173, LINC00096, ZEB2-AS1, HIF1A-AS2, HULC, LUCAT1, SNHG12, MALAT1, HOTAIR, HIF1A-AS2, LINC00096, ADPGK-AS1, and ZEB2-AS1, have also shown importance in diagnosis and prognosis [11,14,30,49][11][17][36][86].
2.3. Importance of lncRNAs in Treatment
lncRNAs affect the response to treatments such as chemotherapy, immunotherapy, and radiotherapy [99][87]. H19 is expressed in patients with TNBC during neoadjuvant chemotherapy and is related to effective clinical outcomes. LINK-A expression is linked to response to pembrolizumab treatment in patients with TNBC because its decreased expression reduces CD8+ T-cell infiltration [59][46]. These lncRNAs act as biomarkers for treatment response in patients with TNBC. LncAFAP1-AS1 expression has been observed in patients with TNBC who received radiotherapy after surgery, and this lncRNA acts as biomarker for radiotherapy [82][69]. Moreover, lncRNAs are involved in angiogenesis. LINC01133 expression is induced by mesenchymal stem/stromal cells that adjoin TNBC cells [33][20]. lncRNAs are actively involved in the regulation of cell proliferation and apoptosis as well as drug resistance in TNBC [16,44,47,61,99][31][34][48][87][88]. DRHC and HOTAIR inhibit TNBC growth and development [31][18]. HOTAIR plays a role in the invasion and migration of TNBC cells and is used as a biomarker for TNBC metastasis in circulation and tissues, indicating poor survival and response [31,32][18][19]. DRHC inhibits TNBC cell proliferation by downregulating the expression of HOTAIR, whereas HOTAIR does not affect the expression level of DRHC. H19 expression is reduced in TNBC cells, whereas PTCSC3 expression is not altered by H19 overexpression [61][48]. HIST2H2BC and SNRPEP4 were identified in 165 frozen tissue samples by transcriptome microarrays; these lncRNAs are involved in taxane chemotherapy in patients with TNBC. Increased miR-377-3p expression delays TNBC progression by regulating the inc00339/miR-377-3p/HOXC6 axis and inhibits TNBC proliferation and apoptosis. Therefore, it is used as therapeutic target. HIF1A-AS2 expression is upregulated in TNBC mammary tissue, which is linked to overall survival. HOTAIR is closely associated with androgen receptor expression and used as a therapeutic strategy to prevent metastasis. The miR-199a/FOXP2 pathway is induced by LINC01133 and triggers the proliferation of TNBC cells. Various lncRNAs act as stem cell markers, such as DANCR, LINC01638, LINC-ZNF469-3, NEAT1, NRAD1, and ASRPS [75,87][62][74]. Some lncRNAs promote vasculogenic mimicry, providing growth supplementation for tumour formation in TNBC. TP73-AS1, which is activated by the miR-490-3p/TWIST1 pathway, is one example. LINK-A alters glycolysis by mediating HIF1α phosphorylation at Tyr565 and Ser7 [3,16,44,47][31][34][88][89]. MANCR inhibits DNA damage and prevents disease progression [66][53]. AWPPH is involved in the prevention of tumourigenesis upon treatment with carboplatin; AWPPH small interfering RNA (siRNA) silencing leads to increased chemosensitivity in TNBC [10,56][43][90]. TUG1 induces the expression of miR-197, reduces the activation of WNT signalling, and enhances TNBC cell sensitivity to cisplatin [75][62]. These findings demonstrate the importance of lncRNAs in the prevention of tumourigenesis. More studies are required to explore lncRNA treatment options. Early studies showed that HOTAIR recruits the polycomb repressive complex 2 to its target genes through the CoREST/REST H3K4 demethylase complex [75][62].
References
- Bissanum, R.; Chaichulee, S.; Kamolphiwong, R.; Navakanitworakul, R.; Kanokwiroon, K. Molecular Classification Models for Triple Negative Breast Cancer Subtype Using Machine Learning. J. Pers. Med. 2021, 11, 881.
- Melone, V.; Salvati, A.; Brusco, N.; Alexandrova, E.; D’Agostino, Y.; Palumbo, D.; Palo, L.; Terenzi, I.; Nassa, G.; Rizzo, F.; et al. Functional Relationships between Long Non-Coding RNAs and Estrogen Receptor Alpha: A New Frontier in Hormone-Responsive Breast Cancer Management. Int. J. Mol. Sci. 2023, 24, 1145.
- Mei, J.; Hao, L.; Wang, H.; Xu, R.; Liu, Y.; Zhu, Y.; Liu, C. Systematic Characterization of Non-coding RNAs in Triple-negative Breast Cancer. Cell Prolif. 2020, 53, e12801.
- Richard, J.L.C.; Eichhorn, P.J.A. Deciphering the Roles of LncRNAs in Breast Development and Disease. Oncotarget 2018, 9, 20179–20212.
- Wang, S.; Ke, H.; Zhang, H.; Ma, Y.; Ao, L.; Zou, L.; Yang, Q.; Zhu, H.; Nie, J.; Wu, C.; et al. LncRNA MIR100HG Promotes Cell Proliferation in Triple-Negative Breast Cancer through Triplex Formation with P27 Loci. Cell Death Discov. 2018, 9, 805.
- Wu, Y.; Wang, Z.; Yu, S.; Liu, D.; Sun, L. LncmiRHG-MIR100HG: A New Budding Star in Cancer. Front. Oncol. 2022, 12, 997532.
- Zhang, M.; Wang, N.; Song, P.; Fu, Y.; Ren, Y.; Li, Z.; Wang, J. LncRNA GATA3-AS1 Facilitates Tumour Progression and Immune Escape in Triple-negative Breast Cancer through Destabilization of GATA3 but Stabilization of PD-L1. Cell Prolif. 2020, 53, e12855.
- Qi, F.; Qin, W.; Zang, Y. Molecular Mechanism of Triple-negative Breast Cancer-associated BRCA1 and the Identification of Signaling Pathways. Oncol. Lett. 2019, 17, 2905–2914.
- Zhang, Y.; Tan, Y.; Wang, H.; Xu, M.; Xu, L. Long Non-Coding RNA Plasmacytoma Variant Translocation 1 (PVT1) Enhances Proliferation, Migration, and Epithelial-Mesenchymal Transition (EMT) of Pituitary Adenoma Cells by Activating β-Catenin, c-Myc, and Cyclin D1 Expression. Med. Sci. Monit. 2019, 25, 7652–7659.
- Tang, J.; Li, Y.; Sang, Y.; Yu, B.; Lv, D.; Zhang, W.; Feng, H. LncRNA PVT1 Regulates Triple-Negative Breast Cancer through KLF5/Beta-Catenin Signaling. Oncogene 2018, 37, 4723–4734.
- Zhang, H.; Zhang, N.; Liu, Y.; Su, P.; Liang, Y.; Li, Y.; Wang, X.; Chen, T.; Song, X.; Sang, Y.; et al. Epigenetic Regulation of NAMPT by NAMPT-AS Drives Metastatic Progression in Triple-Negative Breast Cancer. Cancer Res. 2019, 79, 3347–3359.
- Zhang, W.; Guan, X.; Tang, J. The Long Non-coding RNA Landscape in Triple-negative Breast Cancer. Cell Prolif. 2021, 54, e12966.
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk between Long Non-Coding RNAs, Micro-RNAs and MRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669.
- Ma, L.; Song, G.; Li, M.; Hao, X.; Huang, Y.; Lan, J.; Yang, S.; Zhang, Z.; Zhang, G.; Mu, J. Construction and Comprehensive Analysis of a CeRNA Network to Reveal Potential Novel Biomarkers for Triple-Negative Breast Cancer. Cancer Manag. Res. 2020, 12, 7061–7075.
- Le, K.; Guo, H.; Zhang, Q.; Huang, X.; Xu, M.; Huang, Z.; Yi, P. Gene and LncRNA Co-Expression Network Analysis Reveals Novel CeRNA Network for Triple-Negative Breast Cancer. Sci. Rep. 2019, 9, 15122.
- Liu, Z.; Mi, M.; Li, X.; Zheng, X.; Wu, G.; Zhang, L. LncRNA OSTN-AS1 May Represent a Novel Immune-Related Prognostic Marker for Triple-Negative Breast Cancer Based on Integrated Analysis of a CeRNA Network. Front. Genet. 2019, 10, 850.
- Wang, Y.; Zhang, G.; Han, J. HIF1A-AS2 Predicts Poor Prognosis and Regulates Cell Migration and Invasion in Triple-negative Breast Cancer. J. Cell. Biochem. 2019, 120, 10513–10518.
- Zhao, W.; Geng, D.; Li, S.; Chen, Z.; Sun, M. LncRNA HOTAIR Influences Cell Growth, Migration, Invasion, and Apoptosis via the MiR-20a-5p/HMGA2 Axis in Breast Cancer. Cancer Med. 2018, 7, 842–855.
- Yu, F.; Wang, L.; Zhang, B. Long Non-coding RNA DRHC Inhibits the Proliferation of Cancer Cells in Triple Negative Breast Cancer by Downregulating Long Non-coding RNA HOTAIR. Oncol. Lett. 2019, 18, 3817–3822.
- Tu, Z.; Schmöllerl, J.; Cuiffo, B.G.; Karnoub, A.E. Microenvironmental Regulation of Long Noncoding RNA LINC01133 Promotes Cancer Stem Cell-Like Phenotypic Traits in Triple-Negative Breast Cancers. Stem Cells 2019, 37, 1281–1292.
- Zhao, M.; Zhang, M.; Tao, Z.; Cao, J.; Wang, L.; Hu, X. MiR-331-3p Suppresses Cell Proliferation in TNBC Cells by Downregulating NRP2. Technol. Cancer Res. Treat. 2020, 19, 153303382090582.
- Li, P.; Zhou, B.; Lv, Y.; Qian, Q. LncRNA HEIH Regulates Cell Proliferation and Apoptosis through MiR-4458/SOCS1 Axis in Triple-Negative Breast Cancer. Human Cell 2019, 32, 522–528.
- Yang, F.; Shen, Y.; Zhang, W.; Jin, J.; Huang, D.; Fang, H.; Ji, W.; Shi, Y.; Tang, L.; Chen, W.; et al. An Androgen Receptor Negatively Induced Long Non-Coding RNA ARNILA Binding to MiR-204 Promotes the Invasion and Metastasis of Triple-Negative Breast Cancer. Cell Death Differ. 2018, 25, 2209–2220.
- Tariq, A.; Hao, Q.; Sun, Q.; Singh, D.K.; Jadaliha, M.; Zhang, Y.; Chetlangia, N.; Ma, J.; Holton, S.E.; Bhargava, R.; et al. LncRNA-Mediated Regulation of SOX9 Expression in Basal Subtype Breast Cancer Cells. RNA 2020, 26, 175–185.
- Wang, J.; Xi, C.; Yang, X.; Lu, X.; Yu, K.; Zhang, Y.; Gao, R. LncRNA WT1-AS Inhibits Triple-Negative Breast Cancer Cell Migration and Invasion by Downregulating Transforming Growth Factor Β1. Cancer Biother. Radiopharm. 2019, 34, 671–675.
- Filippova, E.A.; Fridman, M.V.; Burdennyy, A.M.; Loginov, V.I.; Pronina, I.V.; Lukina, S.S.; Dmitriev, A.A.; Braga, E.A. Long Noncoding RNA GAS5 in Breast Cancer: Epigenetic Mechanisms and Biological Functions. Int. J. Mol. Sci. 2021, 22, 6810.
- Han, C.; Li, X.; Fan, Q.; Liu, G.; Yin, J. CCAT1 Promotes Triple-Negative Breast Cancer Progression by Suppressing MiR-218/ZFX Signaling. Aging 2019, 11, 4858–4875.
- Wang, Y.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Guo, B.; Zhang, S.; Wu, R.; Zhang, Z.; et al. LncRNA-Encoded Polypeptide ASRPS Inhibits Triple-Negative Breast Cancer Angiogenesis. J. Exp. Med. 2020, 217, e20190950.
- Tang, M.; Liu, Y.; Zhang, Q.; Zhang, P.; Wu, J.; Wang, J.; Ruan, Y.; Huang, Y. Antitumor Efficacy of the Runx2-dendritic Cell Vaccine in Triple-negative Breast Cancer In Vitro. Oncol. Lett. 2018, 16, 2813–2822.
- Yang, J.; Meng, X.; Yu, Y.; Pan, L.; Zheng, Q.; Lin, W. LncRNA POU3F3 Promotes Proliferation and Inhibits Apoptosis of Cancer Cells in Triple-Negative Breast Cancer by Inactivating Caspase 9. Biosci. Biotechnol. Biochem. 2019, 83, 1117–1123.
- Yang, L.; Wu, X.; Liang, Y.; Ye, G.; Che, Y.; Wu, X.; Zhu, X.; Fan, H.; Fan, X.; Xu, J. MiR-155 Increases Stemness and Decitabine Resistance in Triple-negative Breast Cancer Cells by Inhibiting TSPAN5. Mol. Carcinog. 2020, 59, 447–461.
- Su, J.; Deng, L.; Wang, Y.-D. Roles and Mechanisms of Long Non-Coding RNAs in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 89.
- Tian, Y.; Xia, S.; Ma, M.; Zuo, Y. LINC00096 Promotes the Proliferation and Invasion by Sponging MiR-383-5p and Regulating RBM3 Expression in Triple-Negative Breast Cancer. OncoTargets Ther. 2019, 12, 10569–10578.
- Ma, J.; Zhao, W.; Zhang, H.; Chu, Z.; Liu, H.; Fang, X.; Tang, D. Long Non-Coding RNA ANRIL Promotes Chemoresistance in Triple-Negative Breast Cancer via Enhancing Aerobic Glycolysis. Life Sci. 2022, 306, 120810.
- Tamang, S.; Acharya, V.; Roy, D.; Sharma, R.; Aryaa, A.; Sharma, U.; Khandelwal, A.; Prakash, H.; Vasquez, K.M.; Jain, A. SNHG12: An LncRNA as a Potential Therapeutic Target and Biomarker for Human Cancer. Front. Oncol. 2019, 9, 901.
- Xia, L.; Wang, H. LncRNA LUCAT1/ELAVL1/LIN28B/SOX2 Positive Feedback Loop Promotes Cell Stemness in Triple-Negative Breast Cancer. Breast J. 2022, 2022, 1–12.
- Shi, R.; Wu, P.; Liu, M.; Chen, B.; Cong, L. Knockdown of LncRNA PCAT6 Enhances Radiosensitivity in Triple-Negative Breast Cancer Cells by Regulating MiR-185-5p/TPD52 Axis. OncoTargets Ther. 2020, 13, 3025–3037.
- Wang, R.-X.; Chen, S.; Huang, L.; Shao, Z.-M. Predictive and Prognostic Value of Matrix Metalloproteinase (MMP)-9 in Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer Patients. BMC Cancer 2018, 18, 909.
- Tokumaru, Y.; Katsuta, E.; Oshi, M.; Sporn, J.C.; Yan, L.; Le, L.; Matsuhashi, N.; Futamura, M.; Akao, Y.; Yoshida, K.; et al. High Expression of miR-34a Associated with Less Aggressive Cancer Biology but Not with Survival in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 3045.
- Wang, L.; Luan, T.; Zhou, S.; Lin, J.; Yang, Y.; Liu, W.; Tong, X.; Jiang, W. LncRNA HCP5 Promotes Triple Negative Breast Cancer Progression as a CeRNA to Regulate BIRC3 by Sponging MiR-219a-5p. Cancer Med. 2019, 8, 4389–4403.
- Han, C.; Fu, Y.; Zeng, N.; Yin, J.; Li, Q. LncRNA FAM83H-AS1 Promotes Triple-Negative Breast Cancer Progression by Regulating the MiR-136-5p/Metadherin Axis. Aging 2020, 12, 3594–3616.
- Niu, L.; Fan, Q.; Yan, M.; Wang, L. LncRNA NRON Down-Regulates LncRNA SnaR and Inhibits Cancer Cell Proliferation in TNBC. Biosci. Rep. 2019, 39, BSR20190468.
- Liu, A.; Qu, H.; Gong, W.; Xiang, J.; Yang, M.; Zhang, W. LncRNA AWPPH and MiRNA-21 Regulates Cancer Cell Proliferation and Chemosensitivity in Triple-negative Breast Cancer by Interacting with Each Other. J. Cell. Biochem. 2019, 120, 14860–14866.
- Youness, R.A.; Hafez, H.M.; Khallaf, E.; Assal, R.A.; Abdel Motaal, A.; Gad, M.Z. The Long Noncoding RNA SONE Represses Triple-negative Breast Cancer Aggressiveness through Inducing the Expression of MiR-34a, MiR-15a, MiR-16, and Let-7a. J. Cell. Physiol. 2019, 234, 20286–20297.
- Jin, S.-J.; Jin, M.-Z.; Xia, B.-R.; Jin, W.-L. Long Non-Coding RNA DANCR as an Emerging Therapeutic Target in Human Cancers. Front. Oncol. 2019, 9, 1225.
- Lin, A.; Li, C.; Xing, Z.; Hu, Q.; Liang, K.; Han, L.; Wang, C.; Hawke, D.H.; Wang, S.; Zhang, Y.; et al. The LINK-A LncRNA Activates Normoxic HIF1α Signalling in Triple-Negative Breast Cancer. Nat. Cell. Biol. 2016, 18, 213–224.
- Fu, J.; Dong, G.; Shi, H.; Zhang, J.; Ning, Z.; Bao, X.; Liu, C.; Hu, J.; Liu, M.; Xiong, B. LncRNA MIR503HG Inhibits Cell Migration and Invasion via MiR-103/OLFM4 Axis in Triple Negative Breast Cancer. J. Cell. Mol. Med. 2019, 23, 4738–4745.
- Kansara, S.; Pandey, V.; Lobie, P.E.; Sethi, G.; Garg, M.; Pandey, A.K. Mechanistic Involvement of Long Non-Coding RNAs in Oncotherapeutics Resistance in Triple-Negative Breast Cancer. Cells 2020, 9, 1511.
- Wang, N.; Hou, M.; Zhan, Y.; Sheng, X. LncRNA PTCSC3 Inhibits Triple-negative Breast Cancer Cell Proliferation by Downregulating LncRNA H19. J. Cell. Biochem. 2019, 120, 15083–15088.
- Yao, Z.; Xiong, Z.; Li, R.; Liang, H.; Jia, C.; Deng, M. Long Non-Coding RNA NRON Is Downregulated in HCC and Suppresses Tumour Cell Proliferation and Metastasis. Biomed. Pharmacother. 2018, 104, 102–109.
- Goh, C.Y.; Wyse, C.; Ho, M.; O’Beirne, E.; Howard, J.; Lindsay, S.; Kelly, P.; Higgins, M.; McCann, A. Exosomes in Triple Negative Breast Cancer: Garbage Disposals or Trojan Horses? Cancer Lett. 2020, 473, 90–97.
- Tracy, K.M.; Tye, C.E.; Ghule, P.N.; Malaby, H.L.H.; Stumpff, J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Mitotically-Associated LncRNA (MANCR) Affects Genomic Stability and Cell Division in Aggressive Breast Cancer. Mol. Cancer Res. 2018, 16, 587–598.
- Wang, L.; Liu, D.; Wu, X.; Zeng, Y.; Li, L.; Hou, Y.; Li, W.; Liu, Z. Long Non-coding RNA (LncRNA) RMST in Triple-negative Breast Cancer (TNBC): Expression Analysis and Biological Roles Research. J. Cell. Physiol. 2018, 233, 6603–6612.
- Ferraro, D.A.; Patella, F.; Zanivan, S.; Donato, C.; Aceto, N.; Giannotta, M.; Dejana, E.; Diepenbruck, M.; Christofori, G.; Buess, M. Endothelial Cell-Derived Nidogen-1 Inhibits Migration of SK-BR-3 Breast Cancer Cells. BMC Cancer 2019, 19, 312.
- Kim, E.; Kim, Y.-J.; Ji, Z.; Kang, J.M.; Wirianto, M.; Paudel, K.R.; Smith, J.A.; Ono, K.; Kim, J.-A.; Eckel-Mahan, K.; et al. ROR Activation by Nobiletin Enhances Antitumor Efficacy via Suppression of IκB/NF-ΚB Signaling in Triple-Negative Breast Cancer. Cell Death Dis. 2022, 13, 374.
- Merikhian, P.; Eisavand, M.R.; Farahmand, L. Triple-Negative Breast Cancer: Understanding Wnt Signaling in Drug Resistance. Cancer Cell Int. 2021, 21, 419.
- Wang, P.-S.; Chou, C.-H.; Lin, C.-H.; Yao, Y.-C.; Cheng, H.-C.; Li, H.-Y.; Chuang, Y.-C.; Yang, C.-N.; Ger, L.-P.; Chen, Y.-C.; et al. A Novel Long Non-Coding RNA Linc-ZNF469-3 Promotes Lung Metastasis through MiR-574-5p-ZEB1 Axis in Triple Negative Breast Cancer. Oncogene 2018, 37, 4662–4678.
- Wang, D.; Wang, Z.; Zhang, L.; Sun, S. LncRNA PDCD4-AS1 Alleviates Triple Negative Breast Cancer by Increasing Expression of IQGAP2 via MiR-10b-5p. Transl. Oncol. 2021, 14, 100958.
- Hua, K.; Deng, X.; Hu, J.; Ji, C.; Yu, Y.; Li, J.; Wang, X.; Fang, L. Long Noncoding RNA HOST2, Working as a Competitive Endogenous RNA, Promotes STAT3-Mediated Cell Proliferation and Migration via Decoying of Let-7b in Triple-Negative Breast Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 58.
- Jahangiri, L.; Ishola, T. Dormancy in Breast Cancer, the Role of Autophagy, lncRNAs, miRNAs and Exosomes. Int. J. Mol. Sci. 2022, 23, 5271.
- Tao, W.; Sun, W.; Zhu, H.; Zhang, J. Knockdown of Long Non-Coding RNA TP73-AS1 Suppresses Triple Negative Breast Cancer Cell Vasculogenic Mimicry by Targeting MiR-490-3p/TWIST1 Axis. Biochem. Biophys. Res. Commun. 2018, 504, 629–634.
- Zhou, H.; Sun, L.; Wan, F. Molecular Mechanisms of TUG1 in the Proliferation, Apoptosis, Migration and Invasion of Cancer Cells (Review). Oncol. Lett. 2019, 18, 4393–4402.
- Chen, F.-Y.; Zhou, Z.-Y.; Zhang, K.-J.; Pang, J.; Wang, S.-M. Long Non-Coding RNA MIR100HG Promotes the Migration, Invasion and Proliferation of Triple-Negative Breast Cancer Cells by Targeting the MiR-5590-3p/OTX1 Axis. Cancer Cell Int. 2020, 20, 508.
- Luo, L.; Tang, H.; Ling, L.; Li, N.; Jia, X.; Zhang, Z.; Wang, X.; Shi, L.; Yin, J.; Qiu, N.; et al. LINC01638 LncRNA Activates MTDH-Twist1 Signaling by Preventing SPOP-Mediated c-Myc Degradation in Triple-Negative Breast Cancer. Oncogene 2018, 37, 6166–6179.
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507.
- Li, S.; Zhou, J.; Wang, Z.; Wang, P.; Gao, X.; Wang, Y. Long Noncoding RNA GAS5 Suppresses Triple Negative Breast Cancer Progression through Inhibition of Proliferation and Invasion by Competitively Binding MiR-196a-5p. Biomed. Pharmacother. 2018, 104, 451–457.
- Jin, X.; Xu, X.-E.; Jiang, Y.-Z.; Liu, Y.-R.; Sun, W.; Guo, Y.-J.; Ren, Y.-X.; Zuo, W.-J.; Hu, X.; Huang, S.-L.; et al. The Endogenous Retrovirus-Derived Long Noncoding RNA TROJAN Promotes Triple-Negative Breast Cancer Progression via ZMYND8 Degradation. Sci. Adv. 2019, 5, eaat9820.
- Zhang, K.; Liu, P.; Tang, H.; Xie, X.; Kong, Y.; Song, C.; Qiu, X.; Xiao, X. AFAP1-AS1 Promotes Epithelial-Mesenchymal Transition and Tumorigenesis Through Wnt/β-Catenin Signaling Pathway in Triple-Negative Breast Cancer. Front. Pharmacol. 2018, 9, 1248.
- Imani, S.; Wei, C.; Cheng, J.; Khan, A.; Fu, S.; Yang, L.; Tania, M.; Zhang, X.; Xiao, X.; Zhang, X.; et al. MicroRNA-34a Targets Epithelial to Mesenchymal Transition-Inducing Transcription Factors (EMT-TFs) and Inhibits Breast Cancer Cell Migration and Invasion. Oncotarget 2017, 8, 21362–21379.
- Kong, Y.; Geng, C.; Dong, Q. LncRNA PAPAS May Promote Triple-Negative Breast Cancer by Downregulating MiR-34a. J. Int. Med. Res. 2019, 47, 3709–3718.
- Zhang, G.; Li, H.; Sun, R.; Li, P.; Yang, Z.; Liu, Y.; Wang, Z.; Yang, Y.; Yin, C. Long Non-coding RNA ZEB2-AS1 Promotes the Proliferation, Metastasis and Epithelial Mesenchymal Transition in Triple-negative Breast Cancer by Epigenetically Activating ZEB2. J. Cell. Mol. Med. 2019, 23, 3271–3279.
- Zuo, Y.; Li, Y.; Zhou, Z.; Ma, M.; Fu, K. Long Non-Coding RNA MALAT1 Promotes Proliferation and Invasion via Targeting MiR-129-5p in Triple-Negative Breast Cancer. Biomed. Pharmacother. 2017, 95, 922–928.
- Mofed, D.; Omran, J.I.; Sabet, S.; Baiomy, A.A.; Emara, M.; Salem, T.Z. The Regulatory Role of Long Non-Coding RNAs as a Novel Controller of Immune Response against Cancer Cells. Mol. Biol. Rep. 2022, 49, 11775–11793.
- Zuo, K.; Yuan, X.; Liang, X.; Sun, X.; Liu, S.; Connell, P.P.; Li, X.; Yang, W. QRT-PCR-Based DNA Homologous Recombination-Associated 4-Gene Score Predicts Pathologic Complete Response to Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2022, 191, 335–344.
- Zhang, W.; Yang, S.; Chen, D.; Yuwen, D.; Zhang, J.; Wei, X.; Han, X.; Guan, X. SOX2-OT Induced by PAI-1 Promotes Triple-Negative Breast Cancer Cells Metastasis by Sponging MiR-942-5p and Activating PI3K/Akt Signaling. Cell. Mol. Life Sci. 2022, 79, 59.
- Zhou, Y.; Meng, X.; Chen, S.; Li, W.; Li, D.; Singer, R.; Gu, W. IMP1 Regulates UCA1-Mediated Cell Invasion through Facilitating UCA1 Decay and Decreasing the Sponge Effect of UCA1 for MiR-122-5p. Breast Cancer Res. 2018, 20, 32.
- Manoochehri, M.; Jones, M.; Tomczyk, K.; Fletcher, O.; Schoemaker, M.J.; Swerdlow, A.J.; Borhani, N.; Hamann, U. DNA Methylation of the Long Intergenic Noncoding RNA 299 Gene in Triple-Negative Breast Cancer: Results from a Prospective Study. Sci. Rep. 2020, 10, 11762.
- Zheng, S.; Li, M.; Miao, K.; Xu, H. LncRNA GAS5-promoted Apoptosis in Triple-negative Breast Cancer by Targeting MiR-378a-5p/SUFU Signaling. J. Cell. Biochem. 2020, 121, 2225–2235.
- Shao, H.; Zhu, Q.; Lu, H.; Chang, A.; Gao, C.; Zhou, Q.; Luo, K. HEXIM1 Controls P-TEFb Processing and Regulates Drug Sensitivity in Triple-Negative Breast Cancer. Mol. Biol. Cell 2020, 31, 1867–1878.
- Aranza-Martínez, A.; Sánchez-Pérez, J.; Brito-Elias, L.; López-Camarillo, C.; Cantú de León, D.; Pérez-Plasencia, C.; López-Urrutia, E. Non-Coding RNAs Associated With Radioresistance in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 752270.
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA Interference-Based Therapy and Its Delivery Systems. Cancer Metastasis. Rev. 2018, 37, 107–124.
- Hattab, D.; Bakhtiar, A. Bioengineered SiRNA-Based Nanoplatforms Targeting Molecular Signaling Pathways for the Treatment of Triple Negative Breast Cancer: Preclinical and Clinical Advancements. Pharmaceutics 2020, 12, 929.
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic Delivery of Tumor-Targeting SiRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjugate Chem. 2019, 30, 907–919.
- Wu, B.; Yuan, Y.; Han, X.; Wang, Q.; Shang, H.; Liang, X.; Jing, H.; Cheng, W. Structure of LINC00511-siRNA-conjugated Nanobubbles and Improvement of Cisplatin Sensitivity on Triple Negative Breast Cancer. FASEB J. 2020, 34, 9713–9726.
- Bi, Z.; Li, Q.; Dinglin, X.; Xu, Y.; You, K.; Hong, H.; Hu, Q.; Zhang, W.; Li, C.; Tan, Y.; et al. Nanoparticles (NPs)-Meditated LncRNA AFAP1-AS1 Silencing to Block Wnt/β -Catenin Signaling Pathway for Synergistic Reversal of Radioresistance and Effective Cancer Radiotherapy. Adv. Sci. 2020, 7, 2000915.
- Mou, E.; Wang, H. LncRNA LUCAT1 Facilitates Tumorigenesis and Metastasis of Triple-Negative Breast Cancer through Modulating MiR-5702. Biosci. Rep. 2019, 39, BSR20190489.
- Jordan, N.J.; Dutkowski, C.M.; Barrow, D.; Mottram, H.J.; Hutcheson, I.R.; Nicholson, R.I.; Guichard, S.M.; Gee, J.M. Impact of Dual MTORC1/2 MTOR Kinase Inhibitor AZD8055 on Acquired Endocrine Resistance in Breast Cancer in Vitro. Breast Cancer Res. 2014, 16, R12.
- Du, T.; Shi, Y.; Xu, S.; Wan, X.; Sun, H.; Liu, B. Long Non-Coding RNAs in Drug Resistance of Breast Cancer. OncoTargets Ther. 2020, 13, 7075–7087.
- Marra, A.; Trapani, D.; Viale, G.; Criscitiello, C.; Curigliano, G. Practical Classification of Triple-Negative Breast Cancer: Intratumoral Heterogeneity, Mechanisms of Drug Resistance, and Novel Therapies. NPJ Breast Cancer 2020, 6, 54.
- Hu, X.; Zhang, Q.; Xing, W.; Wang, W. Role of MicroRNA/LncRNA Intertwined with the Wnt/β-Catenin Axis in Regulating the Pathogenesis of Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 814971.
More
