The blood-brain barrier (BBB) is one of the most selective endothelial barriers that protect the brain and maintains homeostasis in neural microenvironments. This barrier restricts the passage of molecules into the brain, except for gaseous or extremely small hydrophobic molecules. Thus, the BBB hinders the delivery of drugs with large molecular weights for the treatment of brain cancers. Various methods have been used to deliver drugs to the brain by circumventing the BBB; however, they have limitations such as drug diversity and low delivery efficiency. To overcome this challenge, microbubbles (MBs)-based drug delivery systems have garnered a lot of interest in recent years. MBs are widely used as contrast agents and are recently being researched as a vehicle for delivering drugs, proteins, and gene complexes. The MBs are 1–10 μm in size and consist of a gas core and an organic shell, which cause physical changes, such as bubble expansion, contraction, vibration, and collapse, in response to ultrasound.
1. Introduction
The blood-brain barrier (BBB) is a unique interface composed of blood capillaries in the central nervous system (CNS) that maintains homeostasis in the neural microenvironment and protects the brain parenchyma from foreign toxic substances
[1][2][3][4][5][6][7][1,2,3,4,5,6,7]. Tight junctions between the brain capillaries and endothelial cells (ECs) prevent the transport of molecules, except for small-sized molecules and lipid molecules, into the brain. In addition to the tight junctions, the brain capillary ECs, which have extraordinarily low transcytosis rates and no fenestrations, inhibit the transcellular transport of macromolecules
[8][9][10][11][12][13][14][15][8,9,10,11,12,13,14,15]. In the treatment of brain cancers, chemotherapy is a major treatment method for suppressing cancer expression to prevent cancer recurrence after surgical resection
[16][17][16,17]. However, the barrier properties of the BBB and efflux transporters P-glycoproteins and breast cancer-resistant proteins that are highly expressed in BBB are the primary cause hindering the delivery of drugs to the CNS for cancer treatment
[12][18][12,18]. Therefore, although several studies have been conducted on approaches for delivering anticancer drugs to the CNS, limitations such as high invasiveness, poor distribution, insufficient efficacy, and intolerable toxicity remain
[12].
Due to the unique structure of the brain, the treatment of brain-related diseases has involved various technological attempts to overcome this hindrance effectively by promoting drug delivery to the CNS
[2][19][2,19]. Among these attempts, thermal ablation using high temperature generated by focused ultrasound energy, whose safety and efficacy have been verified, is being actively attempted worldwide as a surgical treatment for brain diseases such as obsessive-compulsive disorder, mental disorders such as depression, and intractable pain
[20][21][22][23][20,21,22,23]. Particularly, various clinical trials have been conducted to treat brain diseases effectively by promoting drug delivery to the CNS via control of the BBB through the stable and inertial cavitation phenomenon of microbubbles (MBs) that occur when focused ultrasound is applied to the cerebral blood vessels
[24][25][26][24,25,26]. Additionally, the MB-based system is of great interest for the effective delivery of drugs across the BBB due to its non-invasive, transient, reversible, and localized properties
[27][28][29][30][31][32][27,28,29,30,31,32].
In the 1930s, Dr. Karl Dussik first published a paper on the use of ultrasound to visualize cerebral ventricles in the brain by measuring reflections of ultrasound through the head and in the late 1960s, Dr. Claude Joyner first noted the development of MBs as a contrast medium, and the clinical use of MBs started after Gramiak and Shah acknowledged this subject
[33][34][35][33,34,35]. However, the vast potential of MBs as drug-delivery vehicles was recognized only in the late 1990s
[36][37][38][36,37,38]. The structure of the MBs comprises an inner center (core) and an outer layer (shell). MBs physically interact with the surrounding medium through stable cavitation or inertial cavitation, depending on the ultrasound intensity used. On the one hand, stable cavitation generated by ultrasound excitation causes continuous oscillation, which induces a liquid flow around the MBs. On the other hand, inertial cavitation at higher ultrasonic intensities results in the implosion or collapse of the MBs, generating violent mechanical stresses, microjets, and shock waves
[17][39][40][41][42][43][17,39,40,41,42,43]. In addition, since the required acoustic energy and response differ depending on the size and concentration of microbubbles, the physical parameters of ultrasound, such as period per pulse, pulse amplitude, pulse repetition frequency, and exposure length, must be adjusted to determine the optimal ultrasound and microbubble combination
[44][45][44,45]. These phenomena induce biological changes in the BBB, such as increased endocytosis/transcytosis, paracellular passage, and opening of the tight junctions, thus increasing the permeability of the BBB macrostructure for brain cancer therapy
[17][46][47][48][49][17,46,47,48,49].
2. Structure and Composition of MBs
The structure of the MBs comprises the core and shell structures, each with different physicochemical properties. Various materials have been used as the core and shell structural components to increase the stability and efficiency of MBs for brain cancer therapy
[34][50][34,50].
2.1. Core Structure
The first-generation MBs had low stability in solution because atmospheric air constituted the core, and they lacked a stabilizing shell. The stability was increased in the next generation of MBs by incorporating a shell; however, MBs that have an air core have low stability in the biological environment because air dissolves in the blood
[38][51][52][38,51,52]. Therefore, in brain cancer therapy, sulfur hexafluoride (SF
6) or perfluorocarbons, which have higher molecular weights and lower blood solubility than those of atmospheric air, are used as the core in MBs. SonoVue
® (SF
6), Definity
® (perfluoropropane [C
3F
8]), and Optison
® (C
3F
8), which are commercially used, have a perfluorochemical as the material constituting the core. Additionally, other studies have utilized perfluorochemicals such as perfluorobutane (C
4F
10) and perfluoropentane (C
5F
12) as the core components
[38][52][38,52]. Particularly, C
5F
12, a volatile gas with a boiling point of 26 °C, exists in liquid form during the MBs’ manufacturing process and forms the MBs while changing to the gaseous state at body temperature after injection
[34][38][52][53][34,38,52,53].
2.2. Shell Structure
The shell structure comprises polymers, proteins, surfactants, or phospholipids to prevent gas leakage, breakdown, and coalescence
[34][36][53][54][34,36,53,54]. Notably, the shell structure significantly reduces the surface tension of MBs, which is closely related to their stability
[54][55][56][54,55,56]. The water and gas molecules at the MB gas–water interface form hydrogen bonds horizontally along the interface line. This creates a contraction force on the surface of the MBs, which generates surface tension in the inward direction and induces an increase in the pressure inside them. Higher pressure increases the dissolution rate of the gas, reducing the stability of the MBs. The shell structure that covers the surface of the core increases the stability of the MBs by preventing the formation of ordered hydrogen bonds and reducing the inner pressure of the MBs
[28][38][53][56][28,38,53,56].
The constituent shell materials mainly used in the brain cancer therapy system are phospholipids and phospholipid derivatives. Moreover, a broad range of phospholipids with various hydrophobic chain lengths and electrostatic charges have been utilized
[53][57][58][53,57,58]. Thus, it facilitates the customization of the system according to the characteristics of the delivered material. The hydrophobicity of phospholipids affects the drug-loading capacity and stability of the MBs
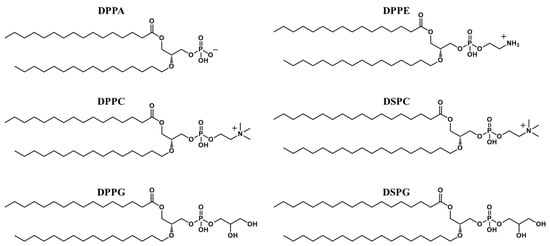
[59]. Moreover, polyethylene glycol-modified phospholipid (PEG-PL) endows the MBs with a stealth effect to evade clearance by the reticuloendothelial system Phospholipids such as dipalmitoyl phosphoric acid (DPPA), dipalmitoylphosphatidylethanolamine (DPPE), dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), distearoylphosphatidylcholine (DSPC), and distearoylphosphatidylglycerol (DSPG) are used to form the shell of the MBs (
Figure 1).
Figure 1. Structure of phospholipids used in microbubble synthesis. (DPPA, dipalmitoyl phosphoric acid; DPPE, dipalmitoylphosphatidylethanolamine; DPPC, dipalmitoylphosphatidylcholine; DPPG, dipalmitoylphosphatidylglycerol; DSPC, distearoylphosphatidylcholine; DSPG, distearoylphosphatidylglycerol).
2.3. Multi-Functionalization of MBs
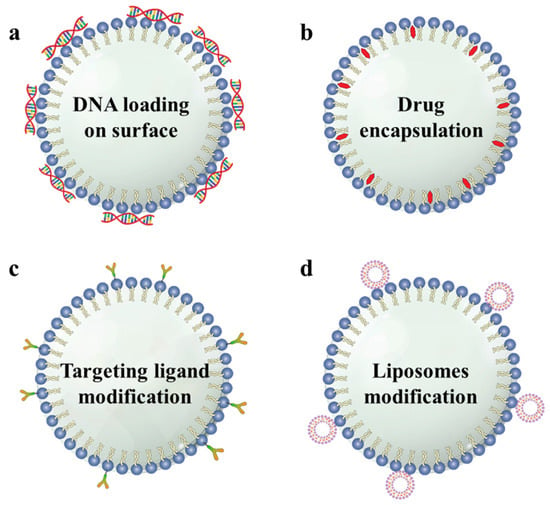
The effects of brain cancer therapy can be enhanced by modifying the MBs comprising a core and shell with cancer drugs, cancer-targeting ligands, DNA, and nanomedicine. These materials develop an association with the MBs shell via electrostatic or hydrophobic interactions, van der Waals forces, physical encapsulation, host-guest interactions, or covalent bonds
[28][38][60][61][28,38,60,61]. Furthermore, functionalized MBs’ structures were designed by modifying the material properties and interactions between the MBs. Hydrophilic DNA is attached to the surface of the MBs comprising positively charged lipid shells via electrostatic interactions (
Scheme 2a)
[62]. Lipophilic or hydrophobic drugs can be encapsulated in the phospholipid shells (
Scheme 2b)
[63][64][65][63,64,65]. The host-guest interaction of avidin-biotin was used to modify the targeting ligand on the MBs’ surface (
SScheme 2c
heme 2c)
[62][65][62,65]. Because the surface area or shell volume of the MBs is restricted, modification of the liposomes on the MBs’ surface provides a larger space for accommodating medicinal materials (
Scheme 2d)
[28][66][67][68][69][70][28,66,67,68,69,70].
Scheme 2. Illustration of the various structures of functionalized microbubbles. (a) DNA can be loaded on the surface of the MBs (b) Hydrophobic drugs can be encapsulated in the shell of the MBs. (c) Targeting ligands can be modified to the surface of the MBs. (d) Drug-loaded liposomes can be attached to the surface of the MBs. (MB, microbubble).