Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tojofaniry Fabien Rakotondrabe and Version 2 by Catherine Yang.
The global increase and prevalence of inflammatory-mediated diseases have been a great menace to human welfare. Several works have demonstrated the anti-inflammatory potentials of natural polyphenolic compounds, including flavonoid derivatives (EGCG, rutin, apigenin, naringenin) and phenolic acids (GA, CA, etc.), among others (resveratrol, curcumin, etc.). In order to improve the stability and bioavailability of these natural polyphenolic compounds, their recent loading applications in both organic (liposomes, micelles, dendrimers, etc.) and inorganic (mesoporous silica, heavy metals, etc.) nanocarrier technologies are being employed.
- polyphenolic
- nanocarriers
- drug delivery
- bioavailability
1. Polyphenolic Nano Delivery for Rheumatoid Arthritis
Rheumatoid arthritis is one of the inflammation-mediated diseases and is characterized by continuous inflammation of the synovial joints, and it can result in lasting sufferance, disability, and even early death [1][58]. The joints start swelling when the synovium is subjected to hyperplasia by an inner proliferation of macrophages and fibroblast cells. Additionally, this hypertrophied synovium invades the adjacent tissues, such as the cartilage and bone surfaces, that enhance inflammatory responses. Therefore, it has been reported that high amounts of pro-inflammatory mediators, such as TNF-α, IL-1, IL-6, and IL-17, are quantified in RA [2][59]. Additionally, this TNF production has been reported to be triggered by the autoantibodies ACPAs. However, these cytokines mitigate the main function of the cartilage-replenishing matrix and chondrocytes through nitric oxide (NO) stimulation, as well as other pro-inflammatory signal releases [3][60].
In terms of flavonoids, a comparison of different solid lipid-based nanocarriers (stearic acid, stearic-lauric, or lecithin-chitosan) loaded with naringenin resulted in the effective activity of this encapsulation within stearic-lauric (Nar-SL). In addition to the extended releases, Nar-SL also diminished the inflammatory factors, together with the degradation of the joint in CFA-induced RA. The complementary anti-inflammatory action between naringenin and lauric acid was supposed to be the principal reason [4][63]. Another study by Mohanty’s research group, in 2020, combined naringin with phenethyl isothiocyanate (PEITC) in a liposome carrier (1,2-dipalmitoyl-sn-glycero3-phosphocholine/cholesterol/1,2-stearoyl-sn-glycerol-3-phosphoethanolamine-020CN (DPPC/Chol/DSPE-020CN)). The intraperitoneal injection of this combinatorial formulation for 3 weeks increased the anti-inflammatory cytokine IL-10 level and attenuated bone erosion. The immune cell infiltrations in Freund’s complete adjuvant (FCA)-induced arthritis were also hindered. A synergistic enhancement of the antiarthritic action of naringenin and PEITC was determined from this preparation [5][64]. The same research group lately investigated the delivery optimization of naringin encapsulated within a biodegradable polymer PLGA (NAR-PLGA-NPs). A sustained release of the loaded compound in the intestine was discovered when stabilizing NAR-PLGA-NPs with poloxamer and sodium dioxylate. In the meantime, a significant increase of the anti-inflammatory marker IL-10, as well as the attenuation of RF and C-reactive protein (CRP) releases, were discovered in arthritic-induced rats [6][65]. A nanocrystal strategy through the wet milling of naringenin with a co-poloxamer (F127) was also applied and resulted in bioavailability improvement and synovial damage, as well. Oral administration of nanocrystal has increased the cellular uptake and diffusion in CIA-induced RA model [7][66].
Metallic nanocarriers, loaded with resveratrol, using ruthenium as the core product and coated with PLGA-dextran sulfate (QRu-PLGA-RES-DS), were developed to improve the bioefficacy of resveratrol in RA. The self-assembly of the formulation has enhanced the bioavailability and circulating time of resveratrol, while triggering macrophage polarization to the M2 phenotype. Meanwhile, M1 macrophage infiltration at the synovia was reduced, which signifies the antiarthritic strength of this elaborate core-shell structure [8][67]. A mixed micelle, built of a mixture of poloxamer 188 and poloxamer 407 coated with poly-lactic acid (PLA), was also designed to load resveratrol for intra-articular injection. One week of the co-micellar injection on CFA-induced RA was enough to reduce the synovium swelling, which correlated with the reduction of TNF-α levels, as well as the cartilage replenishment [9][68]. Combinatorial application of methotrexate and resveratrol (MTX-RSV) nanoemulsion loaded in gel (carbopol 940) showed a greater inhibition of inflammation (78.76 ± 4.16%) and anti-arthritic effects in vivo than those loaded individually [10][69]. Likewise, the oral administration of resveratrol loaded in hydrogel (cellulose aerogel) was demonstrated to exert a great impact on arthritis model management. In addition to the bioavailability amelioration of resveratrol, this delivery system also helped to induce the anti-inflammatory effects by downregulating the P38 pathway and activating SIRT-1 expressions [11][70].
When loaded alone in solid–liquid nanocarriers, curcumin showed promising potential in ameliorating the synovial inflammation of CFA-induced models by reducing immune modulation and inflammatory cascade [12][71]. However, a co-encapsulated formulation of resveratrol and curcumin in lipid nanoparticles showed pronounced antioedematogenic efficiency and cartilage damage protection than the single-loaded standard in CFA-induced models. The lipid core of the product was formed of grape seed oil, and the emulsifier was sorbitan monostearate and polysorbate 80, in which an equal amount of the two phenolic standards were incorporated [13][72]. On the other hand, a HAS-based developed nanoparticle was recently developed to co-deliver curcumin and prednisolone. This latter exerted a more extended circulating time of curcumin before release, due to the attachment of the loaded drug in the albumin mesh. Furthermore, the synergistic anti-inflammation effect of curcumin and prednisolone was discovered with an apparent accumulation at the targeted site of action [14][73]. Another curcumin delivery carrier using gel nanoemulsion was developed for its anti-arthritic effect. The nanoemulsion increased the permeability of the carrier and resulted in a significant decrease of inflammatory markers, notably TNF-α and IL-1β, while its gelation within carbopol-980 enhanced the topical anti-arthritis efficacy on CFA-induced model paw [15][74]. The incorporation of curcumin in polymeric nanocarrier, based on carboxymethyl cellulose acetate butyrate (CUR-CMCAB) resulted in a promising release time and anti-arthritic efficiency when taken orally by CFA-induced rats. These were explained by the complete dispersal of curcumin to its amorphous form in the polymeric shell and the improvements seen from the different behavior testing along the treatment [16][75]. In 2018, Fan et al. entrapped curcumin in hyaluronic acid to form nanomicelle particles, in which the intraperitoneal injection significantly decreased the swelling of CFA-induced arthritis models. A reduced level of VEGF expression and inflammatory cytokines was determined following the treatment. In parallel, it improved the lubrication of joint cartilage in arthritis [17][76]. Kang and coworkers formulated an acid-triggered micelle particle that releases instantly the loaded curcumin once in an acidic environment. In monosodium iodoacetate (MIA)-induced osteoarthritis, the injection of this micelle solution blockaded pro-inflammatory factors expressions, such as IL-1β and TNF-β. In addition, this latter protected the degradation of the cartilage in the synovium [18][77]. Figure 14, below, illustrates some examples of applications of phenolic-loaded nanocarriers applied in rheumatoid arthritis.
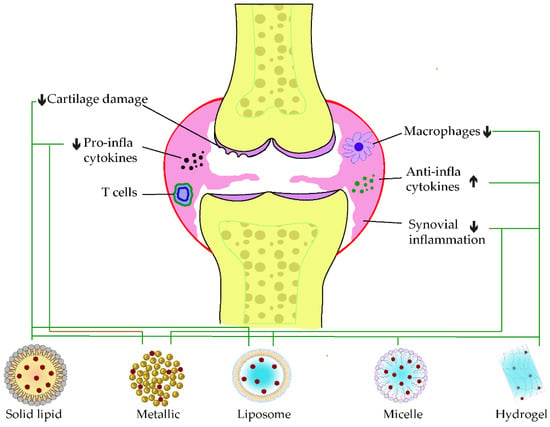
Figure 14. Examples of nanocarrier delivery of polyphenolics and their targets in Rheumatoid arthritis. The black arrows up signify the increasing while the down ones indicate the decreasing effects. The black dots in the figure consist of pro-inflammatory cytokines, the red dots are anti-inflammatory cytokines, and the yellow dots represent the bone marrow. The bold lines match the nanocarriers and their respective targets.
2. Polyphenolic Nano Delivery for Neurodegenerative Disease
Neurodegenerative disease is among the crucial health-threatening issues affecting learning abilities and memory, especially for old people. It is commonly manifested in the form of Alzheimer’s and Parkinson’s diseases. In addition to the abnormal aggregation of neuronal proteins, recent findings have demonstrated that inflammation plays a critical role in the pathogenesis of this disease [19][78]. Alzheimer’s disease (AD) is characterized by the extraneuronal aggregations of amyloid-β (Aβ) proteins, intraneuronal formation of neurofibrillary tangles from hyperphosphorylation of tubulin-associated unit (tau) protein, and neuronal damages [20][79]. Parkinson’s disease (PD) mostly impacts the motor system. The hallmark of this disease consists of a midbrain gradual loss of dopaminergic neurons, accompanied by the intracellular aggregation of α-synuclein (α-syn), also known as Lewy bodies (LB) [21][80]. Likewise, neuro-inflammation caused by microglia plays a pivotal function in the pathogenesis and development of PD.
A great number of delivery systems loaded with phenolic compounds have been pioneered for improving their neuroprotection efficiency and target deliverance. It has been reported that nanoparticles formed with EGCG in PLVA-PEG-PLA (nanoEGCG), when administrated orally, not only improve the bioavailability of EGCG, but also mitigate the release of Aβ plaques, as well as the levels of Aβ1–42, in Alzheimer-induced rats. Additionally, this nanoEGCG was attested to reduce the expression of amyloid precursor proteins, acetylcholinesterase, and glycogen synthase kinase-3 beta (GSK-3β), while the levels of 3-phosphoinositide-dependent protein kinase-1 were found to be rising [22][81]. Another work of Cano and coworkers reported that polymeric nanocarriers built with co-entrapped EGCG and ascorbic acid within PEGlated-PLGA can smoothly pass the blood–brain barrier (BBB) and enhance the neuro-inflammatory effects of EGCG, together with synaptogenesis in APP/PS1 Alzheimer mice. Similarly, the aggregation of Aβ plaques and Aβ1–42 levels declined [23][82]. Given the effectivity of PEGlated-PLGA encapsulation, recent work has mended EGCG loading by co-encapsulation with shRNA, a β-site APP cleaving enzyme antisense (BACE1-AS), and the RGV29 peptide. This nanocarrier formulation sustained the circulation time of the loaded drugs in the bloodstream and facilitated their ability to pass across the BBB. Their neuroprotection effects at the targeted site, the brain, were promising by reducing Aβ levels and mitigating BACE1 function [24][83]. In PD, Li et al., 2020, formulated diverse DSPE-PEG B6-based micelles coated with EGCG (B6ME-NPs). To ease their traceability throughout the deliverance, they incorporated SPIONS in the core shell of the particle. The intravenous administration of these formulations has improved the EGCG stability and delivery to the brain by B6 contribution in overcoming the BBB and inhibited noticeably the aggregation of α-syn agglomeration in vitro [25][84]. Selenoprotein analogs (Tet-1) conjugation of selenium nanoparticles coated with EGCG (Tet-1-EGCG@Se) has also well-enhanced the inhibition of Aβ aggregation and fibrillation in AD. The Tet-1 functionalization was found to facilitate the cellular uptake of the formulation, while Tet-1-EGCG@Se reversed the fibrillation of Aβ agglomerate to its native form [26][85]. A recent investigation showed the effective action of EGCG-Se nanoparticles in neuroprotection via reducing the microglia inflammation in LPS-induced PC12 cells (in vitro) and in the targeted site of a spinal cord injury rat model (in vivo) [27][86].
Rutin loaded in solid lipid nanoparticles has also shown great stability and circulating time in the blood flow. In addition, this nanoparticle can well-infiltrate the BBB, which suggests its efficient brain target delivery [28][87]. Similarly, oral administration of phospholipidic nanocarrier, incorporated with rutin, exhibited eminent brain concentration and attenuated neurological, as well as ischemic, damage in middle cerebral artery occlusion (MCAO) rats [29][88]. A metallic nanocarrier loaded with rutin and coated by the traceable red congo facilitated the localization of Aβ plaques by imaging. Additionally, this theranostic nanoparticle significantly attenuated the cytotoxicity of the Aβ and the release of oxidative products in AD [30][89]. Other antiparkinson’s flavonoids, such as apigenin, were encapsulated in the phospholipid nanocarrier before nasal administration and proved to be delivered well within the brain, while upregulating dopamine releases in haloperidol-induced Parkinson’s models [31][90]. Naringenin nanoemulsion in capryol, tween 20, and water were also highlighted to mitigate amyloidogenesis in Aβ-induced SH-SY5Y by reducing the expression of APP, BACE, and phosphorylated tau in vitro [32][91]. Likewise, the nasal administration of co-loaded nanoemulsion of naringenin and Vit E reversed the MDA levels in 6-OHDA-induced PD rats [33][92]. A polymeric nanocarrier system using modified polycaprolactone was also used to encapsulate naringenin for the treatment of cerebral stroke. It was established that this nanoformulation can impede the release of pro-inflammatory factors and markers in deprived oxygen glucose-induced mesenchyme stem cells (MSC), which suggests their effectiveness in MSC-based ischemic treatment [34][93].
3. Polyphenolic Nano Delivery for Skin Inflammation and Wound
Most dermatological problems are issued by inflammation reactions. However, the inflammation of the skin represents chronic immune-inflammatory diseases involving psoriasis, dermatitis, and lupus erythematosus [35][108]. The psoriatic lesion is specified by epithelial hyperplasia, irregular differentiation of keratinocytes, infiltration of different inflammatory cells in the dermis, and vascularization [36][109]. The occurrence of these keratinocytes and immune cells enhances the release of pro-inflammatory factors around the lesions, which progressively amplify the psoriatic disease [37][110]. Atopic dermatitis, however, is associated with severe pruritus, persistent skin inflammation, and impaired skin barrier function [38][111]. Pathogenesis of this disease includes the upregulation of Th-2-driven inflammation, immunoglobulin E (IgE), and T-cells expressions [39][112].
The application of a nanodelivery system to ameliorate the efficacy of phenolic compounds has gained researchers’ attention during the last decade. Diverse strategies have been applied and achieved more advancement in the exploration of the enhanced method to deliver these kinds of compounds. With regards to EGCG, when entrapped in chitosan polymeric nanoparticles (CHI-EGCG-NPs), it has shown a four-fold effectivity in reducing inflammation responses and the proliferation of cultured keratinocytes. The topical application of this formulation on the imiquimod (IMQ)-induced skin model resulted in a significant decrease in immune cell penetration, as well as diverse psoriasis-related inflammatory markers [40][113]. Similarly, in dermatitis, the encapsulation of this catechin derivative in PEG-PLGA ameliorated the blockading of necroptosis (RIP 1, RIP 3, and MLKL expressions) in the 2,4 dinitrochlorobenzene (DNCB)-induced skin model, while the topical application resulted in noticeable downregulation of inflammatory cytokines releases. In the meantime, the synthesis of MAPK pathways (p-p38, ERK1, ERK2) in the epidermal layers of the dermatitis was also suppressed [41][114]. Co-loading of EGCG with Vit C in chitosan was established to quicken the wound healing of streptozocin-induced mice when administered intraperitoneally. The promotion of collagen deposition and angiogenesis, together with the blockage of immune cell infiltrations in the wounds, was speculated as the main synergistic effects of the co-delivered drugs [42][115]. A hydrogel nanocarrier entrapping EGCG was formulated for wound dressing. When applied to wounded rats, the hydrogel patch showed better wound healing efficacy than the commercial Neuskin® tape, a mechanism which was determined as the regulation of inflammatory and growth factors, together with the promotion of collagen disposition [43][116].
A carbopol-based hydrogel nanocarrier incorporated with rutin nanocrystals was also developed to increase the efficiency of rutin in wound healing. The transdermal application of this preparation showed the protection of epidermal tissues induced by UV radiation [44][117]. An optimized ethosome formulation of rutin in lipid and ethanol has been also proven to protect against skin disorders, through their enhanced anti-inflammatory activity on keratinocytes cells and patient volunteers [45][118]. In the same way, optimal apigenin-loaded ethosome expressed a significant reduction of COX-2 levels in the UV-induced skin mice model [46][119]. Another work, conducted by Pleguezuelos et al., established a liposome formulation loaded with naringenin and tested for biocompatibility in 3T3 fibroblast cells. In vivo assessment demonstrated a better delivery of naringenin in the epidermis of TPA-induced skin mice, as well as great inflammatory-reducing effectivity [47][120]. A chitosan-coated liposome of naringenin was recently developed and proved to ameliorate the wound of experimental rats. The synergistic effects of chitosan and naringenin were stated as the principal therapeutical mechanisms of the optimized product in wound dressing [48][121].
Delivery trials of phenolic acids for skin inflammation have also been conducted. For illustration, the encapsulation of gallic acid in a polymeric nanocarrier composed of a lipid, glycerosome, and poloxamer has increased its deliverance in the skin. More importantly, the topical treatment of TPA-induced skin mice exhibited better anti-inflammatory effects, as explained by the impediment of leukocyte infiltration and activation [49][122]. Co-encapsulation of gallic acid with rutin in polymeric nanovesicle also exhibited positive effects in psoriasis treatment. It acts by blockading the hyperproliferation of keratinocytes, as well as protecting from psoriasis-related inflammation [50][123]. Recent works have established the effectiveness of hydrogel embodiment with gallic acid in wound healing. Topical application of such gallic acid nanovesicle speeds up wound regeneration and demonstrated to downregulate the expression of inflammatory cytokines [51][52][124,125]. Caffeic acid conjugated on nanofiber nanovesicle, formed with PCL and CH, ameliorated its delivery on fibroblast neonatal cell line (NHDF-neo) [53][126]. While conjugated on PLGA nanofiber, caffeic acid expressed better biocompatibility and wound dressing actions in the human fibroblast model [54][127].
Resveratrol was co-loaded with quercetin in lipid nanoparticles (oleic acid-S75) to improve their cellular intakes. When applied topically to a TPA-induced wound in mice, it rapidly regenerated the skin lesion by lowering the permeation of inflammatory cells [55][128]. An optimized co-loading of resveratrol and omega-3 (ω3) in lipid nanosystem has strengthened their anti-inflammatory potential in skin lesion diseases. The said formulation displayed longer circulation time and better inhibition of COX-2 and NO production in LPS-induced cells [56][129]. The incorporation of resveratrol in peptide hydrogel also boosted its wound-healing effectiveness by hindering the release of macrophage cytokines and promoting collagen restructuration [57][130]. Likewise, the hybrid composite encapsulation of curcumin by loading into polymeric (MPEG-PCL) or micelles (PEG-PCL, or PEG-PLA) vesicles before incorporation in hydrogel carriers (chitosan or dextran) resulted in sustained releases with a rapid wound dressing in vivo. Applications of these formulated hydrogels not only triggered the proliferation of fibroblasts, but also enhanced collagen agglomeration and angiogenesis in the tissue lesion [58][59][60][131,132,133]. This system also had positive therapeutic effects in the treatment of psoriasis, in which the pre-encapsulation in nanocarrier was supported to enhance the deliverance of curcumin to the targeted site [61][134]. Adhering curcumin on electrospun nanofibers was also developed for wound healing. Dai and coworkers developed curcumin loaded in gelatin nanofiber and discovered its advantages in inhibiting macrophages, as well as their cytokines releases [62][135]. Moreover, the encapsulation of silver nanoparticles functionalized with curcumin in chitosan was proven to be efficient in reducing wound lesions, without secondary irritation effects in the experimental model [63][136]. Lee and his team have developed a lipid nanocarrier co-loaded with epidermal growth factor and curcumin for treating diabetic chronic wounds. Their finding suggested that, in addition to the potential antioxidant of the formulation, it also helped in triggering the migration of fibroblasts and keratinocytes for wound closure [64][137]. Similar fibroblast migration was also determined when applying silica-based nanoparticles (CU-Si Np) loaded with curcumin on dermal fibroblast cells (HDF fibroblast) [65][138]. The detailed application of these different nanocarriers system loaded with phenolics and their mechanism of actions toward skin inflammation are summarized in Table 12.
Table 12.
Application of phenolic-loaded nanocarriers in skin inflammation.
| Phenolic Compounds |
Type of Nanocarriers (Size) |
Targets | Mechanism of Action | Ref. | |||
|---|---|---|---|---|---|---|---|
| EGCG | Polymeric nanocarrier: PEG-PLGA (176 nm) |
DNCB-induced dermatitis model | Reduces ear and skin thickness, mitigates the inflammatory cytokines releases (TNF-α, IFN-γ, IL-4, IL-6, and IL-17A), blockades necroptosis (RIP1, RIP3, and MLKL expressions), and regulates MAPK pathways (p-p38, ERK1, and ERK2) | [41] | [114] | ||
| EGCG | Polymeric nanocarrier: CHI-EGCG-NPs (211 nm) |
Cultured keratinocytes and IMQ-induced model | Reduces inflammatory responses and modulates psoriasis-related inflammatory cytokines | [40] | [113] | ||
| EGCG | Hydrogel nanocarrier: HG-Ag-EGCG (217 nm) |
Subcutaneous wound in Wistar rats | Accelerates wound properties (modulates growth factors and cytokines) | [43] | [116] | ||
| EGCG | Polymeric nanocarrier: EGCG-Vit C-Gelatin, chitosan (200 nm) |
Wound on STZ-induced diabetic mice | Promotes wound healing by raising collagen accumulation, promoting angiogenesis, and reducing inflammatory cell infiltrations | [42] | [115] | ||
| Rutin | Hydrogel nanocarrier: NC-RU-gel (447 nm) |
UV-induced BALB/c mice | Increases the skin permeability of rutin | [44] | [117] | ||
| Rutin | Ethosomal nanocarrier: ETOH-PL90G-H | 2 | O (112 nm) |
Keratinocyte cells (NCTC2544) and volunteer patients | Improves anti-inflammatory effect | [45] | [118] |
| Apigenin | Ethosomal nanocarrier (36 nm) |
UV-induced skin inflammation | Reduces COX-2 levels | [46] | [119] | ||
| Naringenin | Liposome: NAR-Polysorbate 80-Lipoid | ® | (100 nm) |
3T3 fibroblasts and TPA-induced mice | Reduces skin inflammation | [47] | [120] |
| Naringenin | Liposome: chitosan-coated naringenin nanoemulsion (105 nm) |
Abrasion wound in rat model |
[95][177]. Additionally, curcumin loaded in amphiphilic copolymer (PLGA-PEG-NPs), stabilized by CTAB, impaired the hepatic inflammation of diabetic rats. Orally administered CUR-PLGA-PEG-NPs displayed a lower regulation of COX-2, NF-kB, and TGF-β in STZ-induced diabetic serum, while promoting PPARγ expression [96][178]. Recent research performed a clinical trial delivery of this compound using an oral treatment of an optimized nanomicelle formulation for 12 weeks on patients with metabolic syndromes. The obtained result showed that the treatment has reduced noticeably the plasma triglyceride concentration and HOMA-b index. However, other metabolic syndromes indexes, such as anthropometric features, FBS, and HbA1c, have not been affected [97][179]. Table 23 synthesizes the current application of nanocarriers loaded with phenolic compounds and their actions towards their respective targets in metabolic disorders management.
Table 23.
Application of phenolic-loaded nanocarriers in metabolic disorders.
| Phenolic Compounds |
Type of Nanocarriers (Size) |
Targets | Mechanism of Action | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EGCG | Polymeric nanocarrier: EGCG-PLGA-in hydrogel (112 nm) |
SC model | Increases delivery and reduces cholesterol, LDL-cholesterol while increasing HDL | [83] | [161] | ||||||
| EGCG | Hybrid nanocarrier HG-AG-EGCG |
HFD-induced T2DM C57BL/6 | Improves wound healing in diabetes by suppressing related inflammation | [43] | [116] | ||||||
| EGCG | Protein nanocarrier: EGCG-β-Lg (22 nm) |
HFD obese mice | Lowers triglycerides amount in the model and improved glycemic homeostasis, as well as insulin sensitivity | [82] | [160] | ||||||
| Rutin | Lipid nanocarrier: Lecithin nanophytosome: (72 nm) |
STZ-induced diabetic rats | Mitigates hyperglycemia and hyperlipidemia, reduces the induced damage of the kidney, liver, and pancreas in rats | [85] | [163] | ||||||
| Rutin | Polymeric nanocarrier: ARG-EG-RU (68 nm) |
STZ-induced diabetic rats | Reduces glucose, GHb, and lipid levels, while increasing insulin amount | [84] | [162] | ||||||
| Rutin | Selenium nanocarrier: RU+Se-NPs | Sprague-Dawley rats | Upregulates SIRT-1, Nrf-2, and HO-1 and downregulates JAK-2/STAT3 pathways, as well as inflammatory markers | [86] | [164] | ||||||
| Apigenin | Polymeric nanocarrier: PEGlated-PLGA (160 nm) | Cholecystokinin- induced C57/BL6 mice | Inhibits PSC growth, promotes PSC apoptosis, reduce the expression of PSC-related inflammation | [87] | [165] | ||||||
| Naringenin | Lipid nanocarrier: NRG-Nano (98 nm) |
Methionine choline-induced mice | Improved absorption and showed protection in fatty liver | [89] | [167] | ||||||
| Controls the delivery and ameliorates the wounds construction and skin regeneration | [ | 48 | |||||||||
| Naringenin | ] | [ | 121 | ] | |||||||
| Polymeric nanocarrier: N-PLGA | (129 nm) |
STZ-induced diabetic rat | Ameliorates diabetogenic (increases insulin level, and improves dyslipidemia) | [88] | [166] | Gallic acid | Polymeric nanocarrier: Tween 80-chitosan (330 nm) |
HaCaT cell line | Reduces keratinocyte proliferation and exerts protein protection in vitro. | [ | |
| Gallic acid | Hydrogel nanocarrier: GA-KGM | STZ-induced diabetic rats | Reduces the expression of IL-1β, TNF-α, and COX-2 | 50] | [123] | ||||||
| [ | 98 | ] | [ | 169] | Gallic acid | Hydrogel nanocarrier | Total skin defect model | Enhance wound healing by reducing the expression of IL-6, IL-1β, and TNF-α | |||
| Gallic acid | Polymeric nanocarrier: HAP-PEG-GA-INS (396 nm) | [ | STZ-induced T1D rats | 52] | Reduces blood glucose level in diabetic rats due to a higher delivery of insulin[125] | ||||||
| [ | 99 | ] | [ | 168] | Gallic acid | Hydrogel nanocarrier: GH/GGA | Skin wounded mice | ||||
| Caffeic acid | Lipid nanocarrier: CAPE-loaded-NL (309 nm) |
L-ornithine induced rat | Speeds up the wound healing by scavenging the ROS and promoting tissue regeneration | [51] | Modulated Nrf2 and NF-kB signaling[124] | ||||||
| [ | 100 | ] | [ | 170] | Gallic acid | Polymeric nanocarrier: liposome, glycosome GA polyxomer (70 nm) | TPA induced mice | Improves the skin target delivery and blockades leukocytes infiltration | |||
| Caffeic acid | Lipid nanocarrier: CA–PC (168 nm) | [ | 49 | ] | [122 | HFD-induced hyperlipidemic model] | |||||
| Maintain hepatocyte structure which promotes lipid absorption | [ | 101 | ] | [171] | Caffeic acid | Nanofiber nanocarrier: PLGA | In vitro scratch assay | ||||
| Resveratrol | Lipid nanocarrier: RSV-LPs (215 nm) |
Glucose/STZ-induced β- TC cell | Presents better wound healing properties on human fibroblast | [54] | [127] | ||||||
| Reduces glucose and increased insulin level | [ | 102 | ] | [180] | Caffeic acid | Nanofiber nanocarrier: Chitosan-PCL/CCA | NHDF-neo cell line | Improves cell attachment | [53] | [126] | |
| Resveratrol | Metallic nanocarrier: RES-Au-NPs (20 nm) |
STZ- induced diabetic rats | Overcomes blood-retinal barrier, reduces VEGF-1, TNF-α, MCP-1, ICAM-1, IL-6, and blockades of ERK1/2 signaling pathway | [93] | [175] | Resveratrol | Lipid nanocarrier: Oleic acid-S75 (79 nm) |
TPA induced mice | Neutralizes the inflammatory response | [ | |
| Resveratrol | Polymeric nanocarrier: RES/PEG-PPhe | Intestine of STZ-induced diabetic rats | 55 | ] | [ | Reduces glucose level and increases insulin level while alleviating intestine injury128] | |||||
| [ | 91 | ] | [ | 173] | Resveratrol | Lipid nanocarrier: DSPC/DOPE/ω3 (156 nm) |
RAW 264.7 cell line | Inhibits COX and NO productions | |||
| Resveratrol | Solid lipid nanocarrier: SLN-RES (248 nm) | [ | 56 | ] | [ | STZ-induced rats | Displays hypoglycemic activity, reduces Snap 23, Stx4 and Vamp2 in insulin resistance129] | ||||
| [ | 92 | ] | [ | 174] | Resveratrol | Peptide-hydrogel nanocarrier: Fmoc-FFGGRGD | |||||
| Resveratrol | Rat skin damage model | Inhibits macrophage production of pro-inflammatory cytokines | Polymeric nanocarrier: RSV-PLGA-NPs | [57] | [130] | ||||||
| (176 nm) | OA-induced HepG2 | Promotes lipolysis and mitigates hepatocellular proliferation | [ | 90] | [172] | Curcumin | Hydrogel nanocarriers: PLGA NPS in hydrogel (150 nm) |
IMQ-induced-C57/BL6 mice | Improves anti-psoriasis activity | [61] | |
| Resveratrol | Hybrid nanocarrier: L-Rnano (90 nm) | [ | 134 | ] | |||||||
| HFD-C57BL/6 J mice | Decreases fat mass and inflammation while improving glucose homeostasis | [ | 94 | ] | [176] | Curcumin | Gelatin nanofiber mats | Rat wounded model | Improves the wounds by increasing fibroblast proliferation and migration, inhibiting macrophages, and reducing pro-inflammatory cytokines | [62] | |
| Curcumin | Polymeric nanocarrier: CU-PLA-PEG (117 nm) | [ | 135 | ] | |||||||
| STZ-induced rat | Reduces NF-κB activation, COX-2, TGF-β, and PPARγ expressions | [ | 96 | ] | [178] | Curcumin | Nanocrystal coated nanocarrier: Ch/CNC (Ag NPx/Cury) | Injured rat model | Accelerates wound closure and repairs tissues | [63] | |
| Curcumin | Polymeric nanocarrier: CoQ10-PLGA (115 nm) | [ | 136 | ] | |||||||
| STZ-induced rat | Reduces CRP, IL-6, TNF-α, triglyceride, and total cholesterol levels in plasma. | [ | 95 | ] | [177] | Curcumin | Silica nanocarrier: CU-Si-Nps (36 nm) |
HDF fibroblast cell | Enhances fibroblast migrations | [65] | [138] |
| Curcumin | Micelle nanocarrier: C3-CU-GRAS (12 nm) |
MetS patients for 12 weeks | Curcumin | Nanostructured lipid carrier: EGF–Cur-NLC (331 nm) |
Punched wound on the skin | Accelerates wound closure | [64] | [137] | |||
| Curcumin | Hydrogel nanocarrier: PEG-PLA in dextran hydrogel (65 nm) |
BALB/c mice | Accelerates angiogenesis, fibroblast accumulation, and wound healing | [60] | [133] | ||||||
| Curcumin | Hydrogel nanocarrier: MPEG-PCL in CCS-OA hydrogel (nano: 40 nm) |
Injured tissue | Improves re-epithelialization of the injury | [58] | [131] | ||||||
| Curcumin | Hydrogel nanocarrier: PEG-PCL-PEG in hydrogel (micelle: 26 nm) |
Wound model | Enhances cutaneous repair, and increases collagen content and wound maturity | [59] | [132] |
4. Polyphenolic Nano Delivery in Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a relapsing gastrointestinal tract (GIT) chronic inflammatory that comprehends ulcerative colitis and Crohn’s disease [66][139]. IBD is featured by an irregular immune response. Its pathogenesis is linked with the upregulation of NF-kβ pathways, along with the spectacular recruitment of epithelial and immune cells in the lamina propria [67][140]. Therefore, a rise in the transcription process increases the synthesis of inflammatory mediators involving interleukins (IL-1β, IL-6, IL-12, IL-23) and tumor necrosis factors (TNF-α). In IBD the highly expressed cytokine by T cells is the interferon-γ (IFNγ) that mainly contributes to the blood vessel’s vasculatures, apart from its immunomodulatory action [68][141]. However, the IL-13 cytokine, expressed by the natural killer cells (NK cells), is in charge of the epithelial barrier interruption [69][142]
An advanced technique, by realizing pH response nanovector for delivering rutin to the inflamed intestine, was also established recently. It was constituted by polyglycerol as dendron and dodecyl sulfobetaine as surfactants. The brutal alteration of the environment from the gastric lumen to the mid-alkaline milieu of the intestine triggers the smooth release of rutin from the backbone shell [70][145]. Metallic-based nanoparticles coated with hyaluronic acid (HA) have also been established to load apigenin for targeting colitis. The oral gavage of API-Mn(II)@HA NPs displayed a better restoration of the damaged epithelial colon barrier, reduced inflammatory markers, and enhanced therapeutic effects against DSS-induced colitis mice [71][146].
The silk fibroin nanoparticle was thoroughly used to entrap the stilbene resveratrol for its delivery. In their work, Lozano et al. loaded resveratrol in cocoon source fibroin (RL-FNPs) by adsorption to apply for IBD. Their result emphasized that better anti-inflammatory impacts were found on TNBS model colitis treated with intracolonic injections of RL-FNPs than those treated with coarse resveratrol. The levels of chemokines such as CINC-1, MCP-1, and ICAM-1 dropped in the presence of RL-FNPS, while the peptide amounts in charge of repairing the epithelial barriers increased the mucins, TFF-3, and villin. A recent study used β-lactoglobulin for the encapsulation of this stilbene, which prolonged its release time, when taken orally, and showed greater clinical effects in the IBD mice model. Histological assessment supported that the BLG-RES acts in promoting the expressions of the anti-inflammatory cytokines IL-10 [72][150]. Polymeric nanocarrier, formed with PLGA, galactosamine, and tween 80, was also applied to enhance the oral delivery of resveratrol. This formulation not only increased the intestinal crossing of the loaded drug, but also displayed promising anti-inflammatory effects in RAW 264.7 macrophages [73][151].
Homogenization within high pressure was utilized to stabilize the polymeric D-α-tocopherol PEG 1000 succinate curcumin (TPGS-stabilized curcumin) for its rectal delivery against ulcerative colitis. At first glance, the kinetic profile of the prepared nanoparticle was seven-fold superior to the free curcumin. Furthermore, tremendous anti-inflammatory effects were remarked during the reparation of colitis damages, which were explained by the synergistic effects of the TPGS and the nanosized nature of curcumin [74][152]. A pH-responsive CAP multilayer core-shell nanoparticle system (CAP1AG4CH5@CUNCs) to improve the delivery of curcumin nanocrystals was also developed for ulcerative colitis. The release of the curcumin nanoparticle was sustained, depending on the layer numbers and the colon pH surrounding 7.2. More than ten layers were necessary to ensure the optimal target delivery. Moreover, pronounced re-epithelization of mucosa leading to the hindrance of macrophage infiltrations was found after treating the colitis model with that of a multilayer curcumin-loaded core-shell nanoparticle [75][153]. In Figure 25, some examples of phenolic-loaded nanocarriers with their targets in the management of inflammatory bowel disease are illustrated.
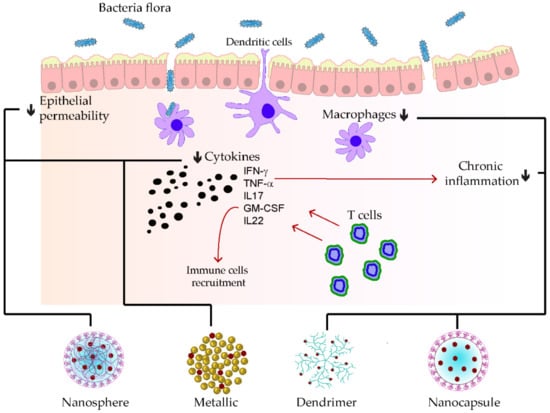
Figure 25. Examples of nanocarrier delivery of polyphenolics and their targets in inflammatory bowel disease. The black arrows up signify the increasing while the down ones indicate the decreasing effects. The red arrows stand for promotions. The black dots consist of inflammatory cytokines. The bold lines match the nanocarriers and their respective targets.
5. Polyphenolic Nano Delivery for Metabolic Disorder
Metabolic disorders, mainly involving diabetes and obesity, are among the life-threatening illnesses of human beings nowadays [76][77][154,155]. Diabetes is manifested by the selective degradation of the insulin producer, pancreatic beta cells, or by insulin resistance or secretion deficiency. When these pancreatic beta cells are inflamed, inflammatory cells are recruited and infiltrate within the pancreatic islets. However, these cells produce inflammatory mediators involving the main cytokines IFNγ, TNF-α, and IL-1β, of which, overexpressions exacerbate the evolution of the pancreatic β-cells inflammation [78][156]. On the other hand, these pro-inflammatory cytokines also contribute to the reduction of insulin sensitivity [79][157]. Insulin resistance strongly impacts lipid metabolism and contributes to the onset of obesity, in general, along with adipose tissue hyperinflammation [80][158]. In obese people, the production of inflammatory mediators is promoted by resident and infiltrated immune cells in the adipose tissue through their MCP-1 stimulations [81][159]
The application of nanocarrier delivery has also received much interest in the management of metabolic diseases. As mentioned in Section 4.3, EGCG has been delivered to quicken the regeneration of impaired wounds in diabetic conditions [42][43][115,116]. Moreover, EGCG loaded in protein nanocarriers with a shell composed of β-lactoglobulin was developed to prevent metabolic syndrome. The administration of the formed nano-complex affects the levels of triglycerides and glycemia in high-fat-fed mice. The insulin sensitivity was also found to be ameliorated, which suggests its potential to reverse the severity of diabetes and obesity [82][160]. Similar therapeutic outcomes were determined while encapsulating EGCG in a hybrid formulation composed of PLGA in a hydrogel. This latter not only merely sustained EGCG releases, but also improved the regulations of LDL and HDL cholesterols in the HFD mice model [83][161].
Site-specific target delivery of rutin within a polymeric carrier composed of ethylene glycol-bis(succinic acid N-hydroxysuccinimide ester) tagged with argpyrimidine ligand (ARG-EG-RU) was also established and assessed with streptozotocin (STZ)-induced diabetic mice. The administration of ARG-EG-Ru considerably diminished the blood glucose levels and GHb in the STZ-induced models, while their insulin releases increased. A decrease in hyperlipidemia was also remarked after the treatment [84][162]. Amjadi et al. used a nanophytosomes system based on lecithin to entrap rutin. Their application in the STZ model resulted in a considerable alleviation of hyperglycemia and hyperlipidemia. In parallel, quick restorations of diabetic-damaged organs, such as the pancreas, liver, and kidney, were also discovered [85][163]. Co-application of rutin with selenium nanoparticles succeeded to improve diabetic nephropathy when administrated orally. It showed relevant cutback of inflammatory markers levels and JAK-2/STAT-3 signaling expressions. Adversely, the levels of SIRT-1 and Nrf-2 were raising, which suggested the renoprotective effects of this combination therapy [86][164]. With regards to apigenin, its delivery by encapsulation in polymeric nanocarrier (PEGlated-PLGA) for managing pancreatitis in a diabetic model has been promising. In addition to its ability to inhibit PSC growth by promoting apoptosis, the formulation also alleviated the overexpression of PSC-related inflammatory mRNAs (IL-6 mRNA, fibronectin mRNA, etc.) [87][165]. In a similar way, naringenin loaded in PLGA (N-PLGA) minimized the amount of glycated hemoglobin and triglyceride levels in STZ-induced diabetic mice after two doses of injections [88][166]. This compound has also been proven to be better delivered when encapsulated within nanoliposome in the treatment of non-alcoholic fatty acid disease (NAFLD), one of the obesity hallmarks [89][167].
When entrapping resveratrol in PLGA through the emulsion method, beneficial therapeutic effects were evidenced, especially in alleviating hyperlipidemia. On oleic acid (OA)-induced Hep G2 cells, in vitro study showed that treatment with RSV-PLGA-NPs enhanced lipolysis of the high-fat cells, which resulted in the clearance of triglyceride accumulations [90][172]. Additionally, a PEGlated phenylalanine encapsulation of resveratrol (RES-PEG-PPhe) downregulated interestingly the level of blood glucose and iNOS overexpression in the STZ-induced diabetic rat model. It also triggered insulin production in the model [91][173]. Other delivery techniques based on lipid shell (dipalmitoylphosphatidylcholine and cholesterol) revealed a considerable increase in insulin degree and expression in STZ-induced β-T cells and further diminished the glucose levels in diabetic models. Similarly, the upregulation of SNARE proteins (Snap23, Stx4, and Vamp2) expressions in the insulin resistance of the STZ-induced model was demonstrated. The delivery system consisted of an oral administration of resveratrol encapsulated in lecithin-palm oil carrier [92][174]. The same diabetic model was used for the evaluation of gold nanocarrier efficiency in delivering resveratrol. Surprisingly, AuNPs ameliorated diabetic retinopathy by lowering the levels of adhered intracellular molecules (ICAM-1), TNF-α, and mRNA expressions and hindered the ERK1/2 signaling pathway [93][175]. The ligand coating of polymeric nanoparticles made of a lipid layer covered with DSPE-PEG5000-peptide (L-Rnano) was delivered properly to the targeted adipose stromal cells (ASCs) of the HFD rat model. The vein injection of the formulated products resulted in a successful diminution of fat and glucose level in the model, while the insulin expressions and obesity-related inflammation were regulated [94][176].
Regarding curcumin, its co-encapsulation with a coenzyme Q10 in PLGA improved its bioavailability. When administrating CoQ10, both diabetic inflammation and lipid metabolism were extenuated in STZ-induced models. Precisely, the synergistic effects of CoQ10 and curcumin in reducing CP, IL-6, and TNF-α, as well as the downregulation of cholesterols were determined
6. Polyphenolic Nano Delivery for Cardiovascular Disease
Cardiovascular disease is one of the silent health burdens that enclose a cluster of disorders attaining the blood vessels and heart. Three main impairments cause this disease, involving atherosclerosis, arterial hypertension, and coronary artery diseases [103][181]. Inflammation plays a substantial role in the pathophysiology of each of these disorders. In atherosclerosis, the damaged arterial vasculature walls are the agglomeration points of lipid mediators. These lipids activate immune cells that trigger a continuous secretion of pro-inflammatory factors and adhesive molecules (VCAM and ICAM). These latter provoke the sustained recruitment of immune cells in the endothelium and enhance the formation of atherosclerotic plaques [104][182]. In coronary artery diseases, diverse mechanisms, such as immune complex-mediated and cell-mediated inflammation, contribute to its growth [103][181]. In addition to the depiction of high rates of inflammatory markers in patients with arterial hypertension, many investigations have also evidenced an abnormal ratio between MMPS and TIMPS [105][183].
With rutin, a silver nanoparticle system (rutin@AgNPs) was established to interrupt the thromboembolism in the vascular system. The release of rutin together with its physiological compatibilities improved considerably, while it prolonged the coagulation times (PT and aPTT). The thrombogenesis formation was also inhibited by injection of this nanoparticle at low doses in vivo [106][187]. Oral administration of nano-lipid vesicle of this biflavonoid also impacted thrombogenesis and clotting formations in the ferric chloride (FC)-induced microvascular model [107][188]. Nanolipid encapsulation of naringenin decorated with folic acid ligands (FA-LNPs/Nrg) expressed higher delivery and anti-atherosclerotic effects in the ApoE-/- model after oral treatment for 3 months. This therapeutic advantage was explained by the ease of FA-LNPs/Nrg to pass across different transmembranes, infiltrate the targeted atherosclerotic cells, and reduce atherosclerotic plaque burdens [108][189]. When co-encapsulating the lipid vesicle with indocyanine green (ICG) and decorated with VCAM-1 molecule to form a theranostic (V-Nar/ICG/LN), other beneficial information arose that supported the effectiveness of the nanoparticle to suppress mRNA expression of inflamed vasculature of different organs [108][189].
Phenolic acid-loaded nanocarrier systems also exhibited enhanced beneficial effects in CVD. He et al. developed gallic acid-loaded polymeric nanovesicles made with PTMC (poly(1,3trimethylene carbonate)) to impede the cytotoxicity of the clinically eluting stent drug used in severe atherosclerosis. Their finding supported that the formulated product improved vein endothelial cells (HUVEC) adhesions, while blockading artery smooth cells (HUASMC) development [109][190]. In addition, anti-platelet aggregation of gallic acid decorated dendrimer (GA-PAMAM) was also established [110][191].
A mesoporous silica nanocarrier was established to embed curcumin compound before treating the myocardial defect induced by doxorubicin. In addition to the delivery improvement of curcumin, mesoporous nanoparticles also enhanced its anti-cardiotoxicity effects by reducing MDA levels and promoting the production of GSH, CAT, as well as SOD in cardiac tissue [111][194]. Synergistic anti-atherosclerotic effects were observed when co-encapsulating curcumin with atorvastatin calcium (Ato) in E-selectin-binding (Esb) ligand-coated liposome (T-AC-Lipo). The decoration with Esb ligands improved the cell delivery of the nanoparticle while curcumin and Ato worked interactively in reducing foam cell generation from monocytes and in blockading adhesions of molecules, such as ICAM-1 and E-selectin [112][195]. Some of these applications of phenolic-loaded nanocarriers and their respective targets for the mitigation of atherosclerosis are shown in Figure 36.
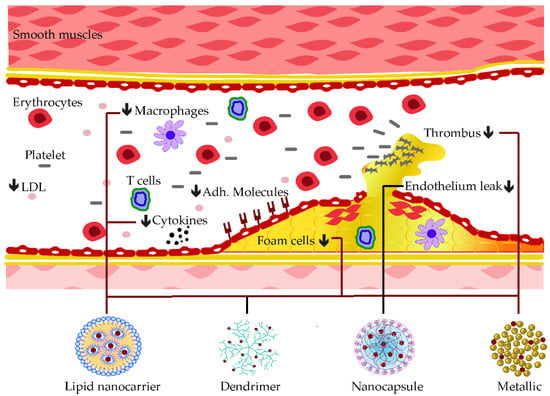
Figure 36. Examples of nanocarrier delivery of polyphenolics and their targets in Atherosclerosis. The black arrows down signify the decreasing effects. The black dots consist of inflammatory cytokines. The bold lines match the nanocarriers and their respective targets.
