Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Hans Henrik Raskov.
As the core component of all organs, the extracellular matrix (ECM) is an interlocking macromolecular meshwork of proteins, glycoproteins, and proteoglycans that provides mechanical support to cells and tissues. In cancer, the ECM can be remodelled in response to environmental cues, and it controls a plethora of cellular functions, including metabolism, cell polarity, migration, and proliferation, to sustain and support oncogenesis. The biophysical and biochemical properties of the ECM, such as its structural arrangement and being a reservoir for bioactive molecules, control several intra- and intercellular signalling pathways and induce cytoskeletal changes that alter cell shapes, behaviour, and viability.
- extracellular matrix
- composition
- cancer
- desmoplasia
- therapeutical targets
1. Introduction
All cells synthesise, secrete, and degrade the extracellular matrix (ECM) occupying the space between them. Apart from being passive mechanical support for cells, the ECM is an extraordinarily complex and highly dynamic macromolecular meshwork of proteins, glycoproteins, proteoglycans, water, minerals, and a multitude of bioactive molecules that determine the phenotypes and molecular functions of the cells it surrounds (Figure 1). The interaction between ECM components, ECM-bound factors and cell surface receptors plays a crucial role in mediating cell adhesion and signalling that regulates multiple biological processes. Additionally, the ECM caters to the three-dimensional architectural structures of organs.
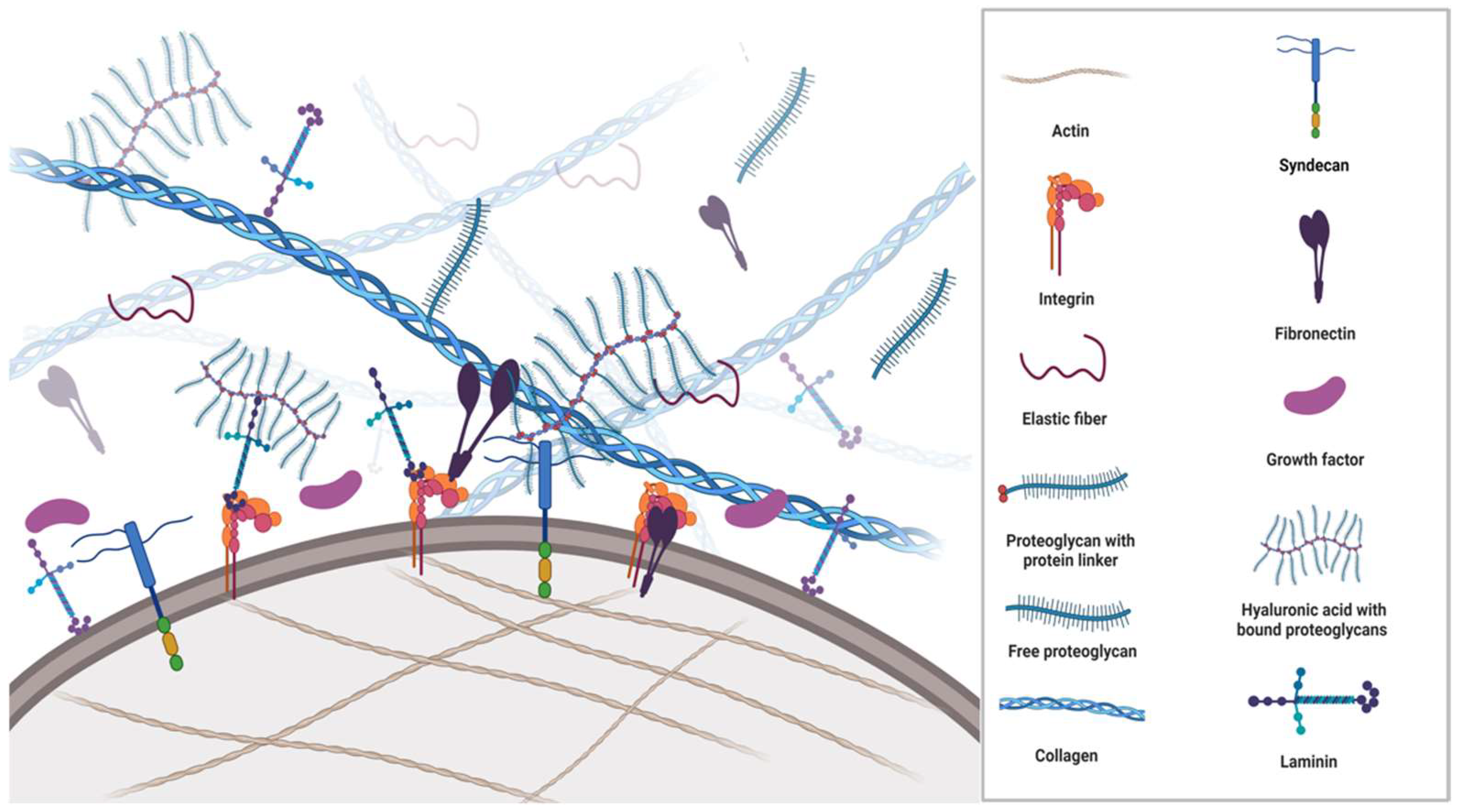
Figure 1. Schematic overview of the ECM structure and its components. The ECM is a complex environment and highly organised support network comprised of multiple proteins such as collagens, fibronectin, proteoglycans, integrins, growth factors and metalloproteinases, which provide cell anchorage. The ECM regulates a plethora of functions to maintain homeostasis. Each matrix protein consists of specific properties that define structural, mechanical, and chemical characteristics. In the figure, a fibronectin molecule is linked to a transmembranous integrin dimer, which is attached to a collagen molecule and thus creates a connection between the cytoskeleton and the ECM. Moreover, laminin complexes are attached to integrins, glycoproteins, and glycolipids through the linker/anchor region (LG domain) on the membrane, creating a dynamic link between cells and the ECM. Syndecans associate with integrins, growth factor receptors, as well as other ECM glycoproteins and collagens. The extracellular domains of syndecans are important for cell–cell and cell–matrix interactions via the glycosaminoglycan sidechains.
Serving as a reservoir for growth factors (GF) and other morphogenic proteins such as matrix metalloproteinases (MMP) originating from tumour cells and non-malignant stromal cells in the microenvironment, the ECM provides positional information to cells and facilitates cell proliferation, differentiation, signalling, and migration. The bidirectional flow of information between cells and the ECM regulates changes in ECM morphology and composition that involves the recruitment and recycling of large numbers of bioactive molecules (reviewed in [1])
The structural scaffolding, mechanical strength, and organisation of ECM components maintain the viability and motility of cells through cell surface contact points that connect the ECM to the cytoskeleton [2,3,4][2][3][4]. The ECM regulates the ability to transport cargo and resist deformation [5,6][5][6].
Through cytoskeleton/ECM adhesions and cytoskeleton contractions, cells can sense the spatial context and mechanical properties of the surrounding ECM. Mechanical stimuli convey downstream signalling (mechano-transduction) that regulates gene transcription [7] and continuously remodel the deposition, degradation, and modification of ECM components. The basement membrane (BM) is part of the ECM that separates epithelial cells from the deeper layers of connective tissues where bioactive molecules moving in and out of cells get filtered. The BM is a pericellular matrix structure that functions as a physical barrier and maintains cellular phenotypes without conferring any structural stability to the cell.
In cancer, tumour cells actively remodel their surrounding ECM through a variety of mechanisms, including the secretion of ECM-degrading enzymes and the synthesis of ECM proteins. The resulting dysregulation induces a range of biophysical and biochemical changes, including excess matrix deposition, rigidity, and fibrosis of the stroma [8]. These changes affect the three-dimensional spatial topology of the matrix around cells as well as cell fate promoting tumour growth, tumour cell migration, metastasis and resistance to anticancer therapies [9,10][9][10]. Understanding the relationship between cell motility and the characteristics of the extracellular matrix (ECM) is of great importance in cancer research, highlighting the necessity of mapping the intricate biological structures of both the ECM and the tumour microenvironment (TME) during the onset and progression of cancer. As wresearchers continue to make significant advancements in exploring intrastromal communication, it is possible that we may o soon witness the implementation of cancer therapies that target specific stromal alterations.
2. Components of ECM
ECM is a composite of cell-secreted macromolecules that include fibrous proteins providing the tissues tensile strength and glycoproteins and proteoglycans providing resistance to compression and deformation. Importantly, these molecules participate in multiple signalling pathways and are described in the subsequent section.3. Fibrous ECM Proteins
3.1. Collagen
Collagen is a polypeptide structure produced by fibroblasts. Except for the brain, collagen is the most abundant protein throughout the human body and the most significant protein in the ECM [11]. Collagen is the major component of most connective tissues supporting and contributing to the three-dimensional from of organs. In addition, collagen plays an important role in various physiologic processes that include angiogenesis, haemostasis, and mineralisation, as well as in common pathologies such as cancer, fibrosis, and cardiovascular diseases [12]. In solid cancers, collagen deposition not only creates a barrier for cytotoxic immune cells and increases therapy resistance but also provides a rich source of exploitable metabolic fuels for cancer cells [13]. Following synthesis and assembly in the endoplasmic reticulum, the precursor peptide procollagen is packaged and exocytosed into the extracellular space. In the extracellular space, propeptide domains at the carboxy- and amino terminals of the procollagen are cleaved off by MMPs to modify the fibril shape and prevent lateral growth. The formation of the mature collagen microfibril requires binding to the N-terminus of fibronectin [14]. Each collagen fibre is made up of several subtypes. Defined by their bonds and amino acid repeats, twenty-eight different types of collagen composed of at least 46 distinct α-chains have been identified in humans [15]. Nearly 50% of amino acids incorporated into collagens are proline and glycine, which have important roles in the regulation of energy production, protein synthesis, redox balance, and intracellular signalling [16]. Collagen can be divided into fibrillar collagens type 1, 2, 3, 5, 11, 24, and 27 and non-fibrillar collagen type 4 (basement membrane); 6 (beaded filaments); 7 (anchor fibres); 8, 10 (short chain); 9, 12, and 14 (fibril-associated collagens with interrupted helices or FACIT); and type 13 (transmembrane collagen). The most abundant is collagen type 1, found in the skin, bones, and tendons [17]. Mutations in collagens 1, 2, 3, 9, 10, and 11 result in a broad range of ailments affecting cartilage, bones, and blood vessels, including osteogenesis imperfecta, various types of chondrodysplasia, Ehlers–Danlos syndrome types 4 and 7, and some cases of osteoarthritis, osteoporosis, and familial aneurysms [18].3.2. Elastin
Elastin is a fibrillar hydrophobic matrix protein that, in contrast to collagen, is able to stretch eight times its resting length [19]. Elastin provides flexibility to blood vessels, skin, lungs, and ligaments. It is synthesised by fibroblasts, vascular smooth muscle cells [20], smooth muscle cells, and several types of epithelial cells. Being the primary ECM protein in arteries, where it amounts to ~50% of the weight [21], it has an impressive ability to withstand the mechanical stress of more than 3 billion expansions and contractions during an 80-year life cycle. The precursor protein, tropoelastin, is secreted with a chaperone molecule that facilitates the correct folding of the protein before it is incorporated into the highly flexible elastin strands. Similar to other ECM proteins, such as collagens, mature elastin is extensively cross-linked with other elastin molecules to form sheets and fibres [22]. Elastic fibres are composed of approximately 90% elastin, whilst the remaining components are primarily comprised of fibrillin glycoproteins. Due to its unique structure, extensive cross-linking and durability, elastin does not undergo significant turnover in healthy tissues where it has a half-life of more than 70 years [23]. It is primarily deposited during prenatal development and childhood and is rarely synthesised during adulthood [24]. Aberrant expression of elastases and degradation of elastin trigger the release of elastokines (fragments of matrix proteins with cytokine-like properties) that promote angiogenesis and regulate cell adhesion, chemotaxis, migration, and proliferation. Much of the elastokine effects are mediated by membrane elastin receptor complexes [23] that trigger signalling pathways involving the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and serine/threonine-protein kinase (AKT) activation [25]. Elastases belong to the enzyme classes of MMP, aspartic proteases, serine proteases, and cysteine proteases. The destruction of elastin promotes the development and progression of different pathological conditions, including chronic obstructive pulmonary disease, atherosclerosis, vascular aneurysms, and cancer. In lung and colon cancer, the degradation of the matrix and fragmentation of elastin was found mainly to occur at the invasive front, and the expression levels of MMP also correlated to the metastatic potential of these cancers [26,27][26][27].4. The Glycoproteins
Glycomics is an important new frontier in life science research. Similar to proteoglycans, glycoproteins are composed of proteins with attached saccharide chains; however, the glycoprotein side chains are much shorter than the saccharide chains in proteoglycans. They contain no (or few) repeating units and are usually branched. The two most important ECM glycoproteins are fibronectin and laminin. Glycoproteins often act as connecting molecules that bind other ECM molecules, GF, and receptors. They have N-linked and O-linked saccharide sidechains, with the N-linked chains being connected to -NH2 on asparagine residues in the protein and O-linked chains to the -OH on the serine/threonine residues. N-linked and O-linked glycoproteins are mainly located on the cell membrane, where they play crucial roles in the cell–cell communication, adhesion, migration, proliferation and healing processes; they may also exist as secreted proteins.4.1. Fibronectin
Within the body, fibronectin exists as soluble plasma glycoproteins (synthesised by hepatocytes and secreted into the blood) and as insoluble cellular fibronectin (a fibrillar cross-linked structure on the cell membranes). It is responsible for cell adhesion, proliferation, migration, and the deposition of ECM proteins [28]. The basic structural unit of fibronectin is a dimer composed of two nearly identical polypeptide chains linked by a pair of disulphide bonds. Fibronectin fibrils serve as mechanical links between the cytoskeleton and the surrounding ECM. It primarily binds to actin-anchored integrins on the cell membrane (Figure 1). Mediating the adhesion of BM components to ECM structures, integrins are heterodimeric (α and β subunits) cell-surface receptors and bi-directional transducers of biochemical signals and mechanical forces acting on the ECM. The α and β subunits both have a cytoplasmic tail, a transmembrane domain, and a large extracellular domain that bind numerous ECM ligands. The anchorage to the ECM is required for normal cells to enter the S phase, even in the presence of GF. If cells detach from their integrin ligation points and lose the sense of their mechanical environment, they undergo a specific type of apoptosis, anoikis (Greek for homeless). Resistance to anoikis is a characteristic feature of tumour cells that enables them to survive under non-adherent conditions [29,30,31][29][30][31]. The connection between the ECM and cytoskeleton stimulates cell proliferation and angiogenesis through pathways that include ERK 1/2 phosphorylation, dysregulation of the HIPPO (tumour suppressor) pathway, and suppression of apoptosis through the nuclear factor kappa B (NF-κB) or the phosphoinositide 3-kinase (PI3kinase)/AKT pathway [32]. Fibronectin fibrillogenesis is initiated by cytoskeleton-derived tensional forces transmitted across transmembrane integrins, typically α5β1 [33]. During this process, soluble molecular fibronectin is irreversibly assembled into insoluble fibrils that stretch up to four times their resting length, which implies domain unfolding and subsequent ECM remodelling [34]. Fibronectin fibres are proposed to be held together by hydrogen and disulphide bonds; however, catalytic agents such as thermolysin, plasmin, thrombin, trypsin, cathepsin D, and chymotrypsin can cleave them. Fibronectin fibrillogenesis and collagen fibrillogenesis have a complex relationship, with fibronectin regulating the assembly of collagen and vice versa [35]. How the production, organisation and matrix deposition of fibronectin are regulated by tumour cells is less understood as the turnover of fibronectin is largely unexplored [36]. Interacting with other ECM proteins, including GF, glycosaminoglycans, cell surface receptors and other fibronectin structures, fibronectin provides key mechanical and chemical signals to induce differentiation and epithelial-mesenchymal transition (EMT) [37]. Transforming growth factor β (TGFβ), fibroblast growth factor (FGR), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) have multiple binding sites within fibronectin. The binding of TGFβ1 to fibronectin fibrils was shown to upregulate EMT [37], whereas dysregulation of fibronectin promoted tumorigenesis and fibrosis, with the expression levels of fibronectin being significant prognostic factors in several cancers [38,39][38][39]. Hypoxia-induced factors upregulated in tumour cells stimulate endogenous FN synthesis. Intercellular signalling between tumour cells and protumorigenic stromal cells, such as tumour-associated macrophages, cancer-associated fibroblasts, and myeloid-derived suppressor cells drive persistent FN deposition and remodelling of the ECM that facilitate growth and dissemination [40,41,42][40][41][42].4.2. Laminin
Laminins are one of the major glycoproteins in the basement membranes that glue cells and tissues together and regulate cellular activities and signalling pathways. Structurally, laminins are cross-shaped, trimeric glycoproteins of 400–800 kDa in size and composed of a few distinct domains, of which 16 different combinations have been identified. Primarily involved in tissue repair and wound healing [43[43][44][45],44,45], all laminin complexes have a high affinity for GF through their heparin-binding domains; thus, apart from contributing to the anchoring of cells, laminin is a storage facility for GF whose release determines cell differentiation, survival, shape, and motility [46]. In hepatocellular carcinoma, laminin was found to be involved in EMT and disease progression [47]. The association between ECM proteins and GF is shown in Table 1.Table 1.
Growth factors and their association with ECM proteins.
| GF | ECM Protein | Growth Factor Functions |
|---|---|---|
| TGFβ | Type IV collagen Heparin/HS Fibronectin Fibrin/fibrinogen Betaglycan Decorin |
Modulates cell growth and differentiation. Stimulates the synthesis of collagen, fibronectin and other ECM components, including HA, TSP, and tenascin. Increases production of protease inhibitors. Reduces the synthesis and secretion of proteases. |
| HGF | Type I, III, IV, V, and VI collagen Heparin/HS Fibronectin Fibrin/fibrinogen |
Stimulates matrix remodelling and epithelial regeneration. Inhibits fibrosis. |
| IGF | IGF-binding protein | Stimulates cell mitogenesis, differentiation, and survival. Amplifies activity through the engagement of integrins or ECM glycosaminoglycans, heparin-binding domains, affecting cell adhesion and migration. |
| PDGF | SPARC Heparin/HS Fibronectin Fibrin/fibrinogen |
Regulates angiogenesis. Attracts fibroblasts and monocytes and accelerates granulation tissue formation and ECM deposition. |
| VEGF | Collagen Heparin/HS Fibronectin Fibrin/fibrinogen |
Controls blood vessel formation and growth. Binds to fibronectin to synergistically promote endothelial cell proliferation. |
| EGF | Collagen Fibronectin |
Stimulates epithelial cell proliferation. Regulates a subset of G1 cell-cycle events. Elevates levels of EGFR. |
| FGF | Heparin/HS Fibronectin Fibrin/fibrinogen |
Induces fibroblast proliferation and angiogenesis. Oligomerisation prolongs activity protection from proteolysis and endocytosis. |
EGF: epidermal growth factor; EGFR: EGF-receptor; FGF: fibroblast growth factor; HA: hyaluronic acid; HGF: hepatocyte growth factor; HS: heparin sulphate; IGF: insulin-like growth factor; PDGF: platelet-derived growth factor; SPARC: secreted protein acidic and rich in cysteine, TGFβ: transforming growth factor beta; VEGF: vascular endothelial growth factor.
 HA turnover occurs in various molecular weights of HA distinguished by their polymer length, e.g., high-molecular-weight HA (Mw > 1.8 × 106 Dalton) and low-molecular-weight HA, Mw 4–10 × 105 Dalton), The molecular weight of HA determines its activities. High-molecular weight HA inhibits mitogenic processes and possesses anti-inflammatory effects, whereas low-molecular weight-HA shows protumorigenic effects by enhancing proliferation and inflammation [58].
Within a solid tumour, HA is deposited by cancer-associated fibroblasts (CAF) and cancer cells and is a major structural component of the TME [59].
HA plays a central role in cancer cell proliferation and migration. HA recruits tumour-associated fibroblasts, macrophages, and HA fragments to promote angiogenesis and immunosuppression, either directly or through macrophage protumorigenic polarisation [60,61][60][61]. Moreover, increased HA levels in the TME are associated with poor prognosis and survival in several cancers [54,62][54][62]. The recruitment and polarisation of macrophages may be used in future targeted anticancer therapies. Chimeric antigen receptors (CARs) have become a promising approach to increasing tumour cell recognition by cytotoxic immune cells. CAR-T cells are already in clinical use. In vitro, tumour-associated macrophages engineered with CAR constructs can be directed at tumour antigens and kill tumour cells by phagocytosis. Furthermore, the CAR-HER2-CD147 construct activates the expression of matrix metalloproteinases, degrades the ECM, and overcomes the physical barrier that prevents the infiltration of cytotoxic immune cells. As the degradation of the ECM may also support the dissemination of tumour cells, any treatment involving metalloproteinases must be calibrated meticulously [63].
Second-generation CAR-M cells are in development. In addition to maintaining the characteristics of first-generation CAR-M technology, the goals of second-generation therapies comprise improving tumour-associated antigen presentation and T-cell activation [64].
HA turnover occurs in various molecular weights of HA distinguished by their polymer length, e.g., high-molecular-weight HA (Mw > 1.8 × 106 Dalton) and low-molecular-weight HA, Mw 4–10 × 105 Dalton), The molecular weight of HA determines its activities. High-molecular weight HA inhibits mitogenic processes and possesses anti-inflammatory effects, whereas low-molecular weight-HA shows protumorigenic effects by enhancing proliferation and inflammation [58].
Within a solid tumour, HA is deposited by cancer-associated fibroblasts (CAF) and cancer cells and is a major structural component of the TME [59].
HA plays a central role in cancer cell proliferation and migration. HA recruits tumour-associated fibroblasts, macrophages, and HA fragments to promote angiogenesis and immunosuppression, either directly or through macrophage protumorigenic polarisation [60,61][60][61]. Moreover, increased HA levels in the TME are associated with poor prognosis and survival in several cancers [54,62][54][62]. The recruitment and polarisation of macrophages may be used in future targeted anticancer therapies. Chimeric antigen receptors (CARs) have become a promising approach to increasing tumour cell recognition by cytotoxic immune cells. CAR-T cells are already in clinical use. In vitro, tumour-associated macrophages engineered with CAR constructs can be directed at tumour antigens and kill tumour cells by phagocytosis. Furthermore, the CAR-HER2-CD147 construct activates the expression of matrix metalloproteinases, degrades the ECM, and overcomes the physical barrier that prevents the infiltration of cytotoxic immune cells. As the degradation of the ECM may also support the dissemination of tumour cells, any treatment involving metalloproteinases must be calibrated meticulously [63].
Second-generation CAR-M cells are in development. In addition to maintaining the characteristics of first-generation CAR-M technology, the goals of second-generation therapies comprise improving tumour-associated antigen presentation and T-cell activation [64].
Integrins activate various intracellular signalling pathways that promote cell survival, growth, and proliferation and targeting these molecules could be an effective strategy to strike at tumour cells. Integrins play key roles in various other diseases, such as ulcerative colitis, cardiovascular diseases and osteoporosis, but they have not been targeted extensively (reviewed in [76]). Kindlin-2 is a widely expressed protein that is critical for integrin-mediated cell–ECM adhesion and signalling. Kindlin-2 localises to the adhesion sites where the ECM molecules are connected to the actin cytoskeleton and increase proline synthesis through interaction with pyrroline-5-carboxylate reductase 1 (PYCR1). PYCR1 is a key mitochondrial enzyme that facilitates the last step in glutamine-to-proline conversion, and its overexpression of PYCR1 is involved in the progression of several cancers, including breast and lung cancer [77]. The increased proline synthesis is linked to increased production and stiffness of the ECM and plays an important role in tumourigenesis [78].
5. Proteoglycans
The proteoglycans (mucoproteins) are composed of a protein core covalently attached to glucosaminoglycans (mucopolysaccharides), such as chondroitin sulphate, heparan sulphate or keratan sulphate (Figure 1). Proteoglycans have excellent water retention, gel-forming and space-filling functions [48], conveying resistance to compression and deformation to cells. Although one of the least abundant components in the ECM, they are integral in maintaining a healthy ECM. Syndecans are transmembrane proteoglycans with heparan and chondroitin sulphate chains attached to their extracellular domain. They may also exist as soluble extracellular domains. Similar to many proteoglycans, they interact with a multitude of ligands, such as GF, adhesion receptors, proteinases, cytokines, chemokines and other ECM proteins to initiate downstream signalling responsible for proliferation, adhesion, angiogenesis, and inflammation [49]. Elevated levels of syndecan expressions in cancer can be correlated with poor outcomes, e.g., of Syndecan-1 in breast cancer and of Syndecan-2 in colorectal cancer, where it is highly associated with metastasis [50].Hyaluronic Acid
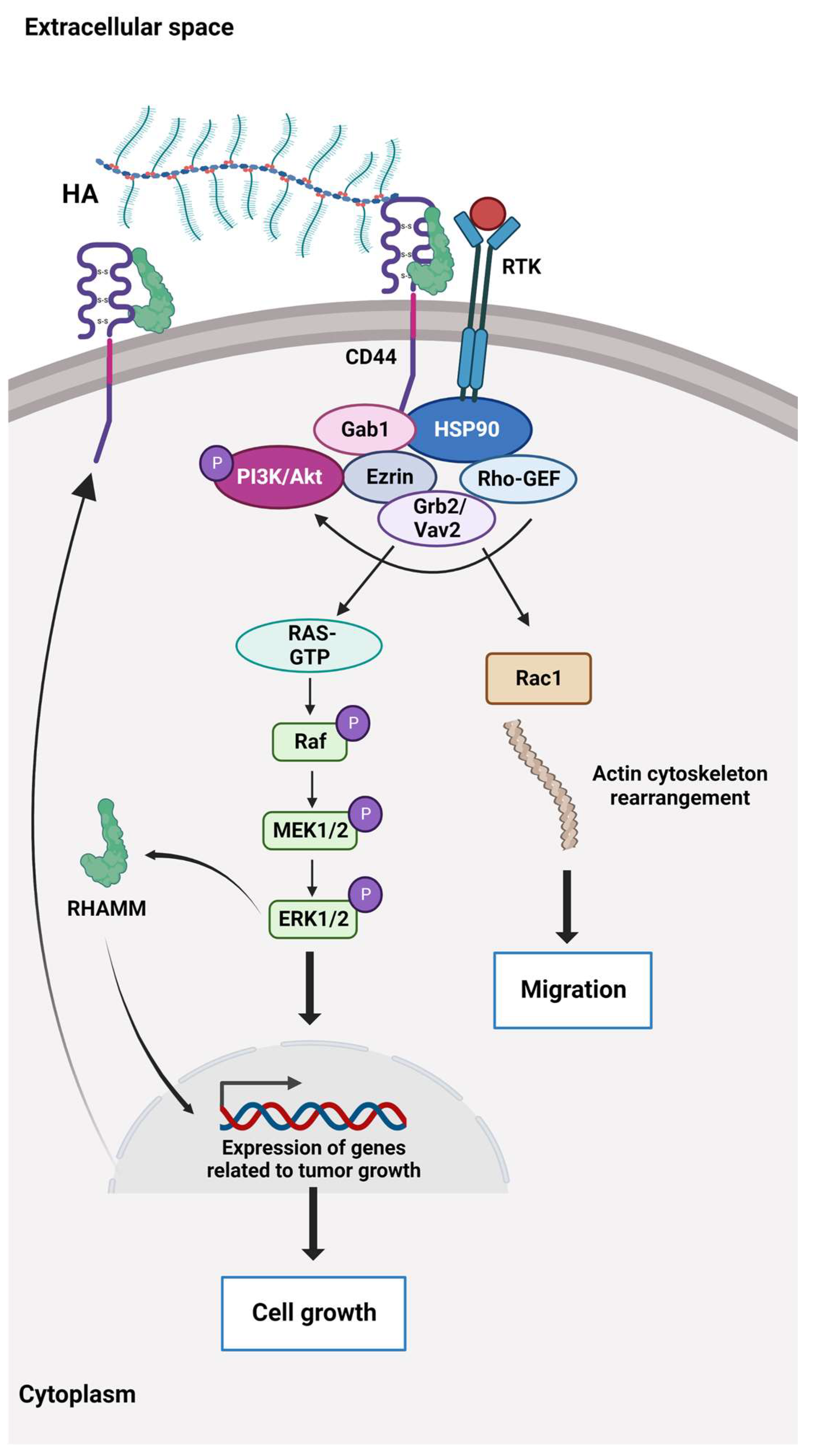
Hyaluronic acid (HA or hyaluronan) is a hydrophilic glycosaminoglycan. HA synthases in the cell membrane mediate the alternate addition of glucuronic acid and N-acetylglucosamine in a growing chain of thousands of disaccharides [51] that are translocated out of the cell during biosynthesis. HA is abundantly present in the ECM of weight-bearing joints and the interstitial gel [52,53][52][53]. HA binds to the cluster of differentiation 44 protein (CD44), a transmembrane receptor that participates in many physiological and pathological processes by interacting and activating key signalling cascades (Figure 2). Ligation of CD44 initiates the expression of genes related to tumour growth, proliferation, and survival, and its ligation with HA induces cytoskeletal rearrangements and membrane ruffling that leads to active cell migration [54,55][54][55]. Further, CD44 serves as a marker for several types of stem cells [56,57][56][57].
Figure 2. The HA-dependent CD44 signalling. CD44 and the receptor for hyaluronic acid (HA)-mediated motility (RHAMM) are the main HA receptors. They are commonly overexpressed in cancer, where they activate signalling pathways related to disease progression. CD44 also interacts with other ligands, such as collagens and matrix metalloproteinases, and as such, a multifunctional receptor mainly involved in proliferation, differentiation, migration, and angiogenesis. In contrast to membrane-bound receptors containing signalling domains, RHAMM (CD168) does not contain signal sequences. RHAMM is localised inside the cell and is exported to the cell surface in response to stimuli such as cytokines, including TGF-β. Extracellularly, RHAMM associates with CD44, and when HA binds to these cell surface receptors, it triggers several signalling pathways and complex formation between CD44 and its co-receptors and increases the expression of TGF-β receptors. Activation of downstream effectors, e.g., Akt, PI3K, MEK1/2, ERK1/2, and Ras/Raf/Rac, results in the expression of a variety of inflammatory cytokines and activation of a feedback loop augmenting cell surface expression of CD44/RHAMM. The CD44/RHAMM complex stimulates cell motility and increases angiogenesis by promoting the migration of endothelial cells towards the tumour. Further, these signalling events drive proliferation, invasion, and cytoskeletal rearrangements, leading to normal cell functions, such as fibroblast migration and immune cell function, and tumour growth and progression. ERK1/2: Extracellular signal-regulated protein kinases 1 and 2, Ezrin: kinase substrate protein, Gab1: PAR-1 kinase, Grb2/Vav2: growth factor receptor-bound protein2/guanine nucleotide exchange factor, GTP: Guanosine triphosphate, HSP90: Heat shock protein 90, MEK1/2: Mitogen-activated protein kinases 1 and 2, PI3K/Akt: Phosphoinositide 3 kinase/protein kinase B (originally Ak strain transforming kinase), Ras/Raf/Rac: Rat sarcoma virus protein/rapidly accelerated fibrosarcoma protein kinase/Ras-related C3 botulinum toxin substrate 1, Rho-GEF: Rho-guanine nucleotide exchange factors, RTK: Receptor tyrosine kinase.
6. Sensing and Communication in ECM
Mechanotransduction allows living organisms to receive and respond to mechanical forces from the internal and external environment. Mechanically activated ion channels represent the primary mechanism for mechanotransduction that effectively converts mechanical stimuli into electrochemical signals. In the cell membrane, the transmembrane and mechanotransducing Piezo proteins (1 and 2) form ion channels that are activated by pressure. When pressure is applied to the cell membrane, the large, three-bladed propeller-shaped molecular complexes flatten and stretch, whereby the ion channels open and allow a flow of calcium and other positively charged ions through the central pore module into the cell, whereby biochemical signals are created. Cells probe their environment mechanically via lamellipodia (membrane protrusions composed of a dense and dynamic network of actin filaments). Lamellipodia decode the mechanical feedback and resistance from their surroundings [65] through membrane integrins and syndecans. These molecular complexes trigger intracellular signalling cascades involving the unfolding of proteins associated with the contractile actin cytoskeleton, such as Rho-associated protein kinase [66]. When the actin protein polymerises to form filaments, it enables the cell to control shape and mechanics—as used by crawling and phagocytising immune cells and migratory tumour cells. In cancer, the cytoskeleton signalling reduces the number of intercellular adhesion molecules, induces EMT, upregulates membrane integrins, and enhances the migratory potential [7] and therapeutic resistance [67]. The mechanotransduction that arises from changes in ECM stiffness shifts cytoskeletal dynamics and releases mechanosensitive molecules such as vinculins, paxicillins, and talins that also regulate adhesion and migration [68,69][68][69]. Mechanosensitive cytoplasmic proteins connect integrin complexes to the nucleus through linker protein complexes that allow a direct transmission from ECM to the nucleus as reviewed in [70]. The mechanisms leading to gene transcription are not mapped fully. Yes-associated protein 1 (YAP) and WW-domain-containing transcription regulator 1 (TAZ) are transcriptional coactivators that are translocated to the nucleus together with β-catenin upon cytoskeletal tension [71,72,73][71][72][73]. YAP/TAZ activation upregulates the genes associated with proliferation and dedifferentiation, and the nuclear translocation of β-catenin directly destabilises intercellular adhesions, all contributing to EMT for migration through the ECM [74,75][74][75]. Simultaneously, the levels of several integrins are upregulated in several tumours. Many signalling pathways are affected by ECM dysregulation, as shown in Table 2.Table 2. A non-exhaustive table summarising the expression levels of some of the main signalling pathways in various solid cancers that are affected by ECM dysregulation, such as aberrant collagen deposition, increased HA expression, and abnormal expression of laminin and fibronectin. It is important to note that the expression levels of signalling pathways may vary. They are not mutually exclusive, and as shown, they often overlap and interact with each other. Signalling pathways are also affected by the accumulation of genetic mutations and disease progression and may change their oncogenic drive. With progression, changes in the composition and organisation of the ECM may affect additional pathways. For example, in early-stage breast cancer, changes in collagen I and IV deposition can activate the PI3K/AKT and MAPK/ERK pathways, whereas in advanced stages, the deposition of different types of collagens, such as collagen VI, can activate the TGF-β pathway. Similarly, in lung cancer, the expression of hyaluronan is increased in advanced stages, leading to dysregulation of the Hippo pathway, which promotes tumour growth and metastasis. CRC: colorectal cancer, EMT: epithelial-mesenchymal transition, EGFR: epidermal growth factor receptor, ERK: extracellular signal-regulated protein kinase, FAK: focal adhesion kinase, HA: hyaluronic acid, HCC: hepatocellular carcinoma, MAP/ERK: microtubule-associated protein kinase; MAPK: mitogen-activated protein kinase, NSCLC: non-small cell lung cancer, PDAC: pancreatic ductal adenocarcinoma, PI3K/Akt: phosphoinositide 3 kinase/protein kinase B (originally Ak strain transforming kinase), Ras/Raf: rat sarcoma virus protein/rapidly accelerated fibrosarcoma protein kinase, RhoA/ROCK: Rho GTPase A/serine-threonine protein kinase, SMAD: Suppressor of Mothers against Decapentaplegic, Src: non-receptor cytoplasmic tyrosine kinase, YAP: Yes-associated protein, TGF-β: transforming growth factor. Wnt: wingless-related integration site.
| Signalling Pathways Affected by ECM Dysregulation | Breast Cancer | NSCLC | CRC | PDAC | Prostate Cancer | Ovarian Cancer | HCC | Glioblastoma | Malignant Melanoma |
|---|---|---|---|---|---|---|---|---|---|
| EMT | + | + | + | + | + | + | |||
| EGFR | + | + | + | ||||||
| FAK | + | + | + | ||||||
| FAK/Src | + | + | + | + | + | ||||
| HA | + | ||||||||
| Hedgehog | + | + | + | + | + | ||||
| Hippo | + | + | + | ||||||
| Hippo/YAP | + | ||||||||
| Integrin | + | + | + | + | + | + | + | + | |
| MAPK/ERK | + | + | + | + | |||||
| Notch | + | + | + | ||||||
| PI3K/AKT | + | + | + | + | + | + | + | + | + |
| RAS/RAF/MAPK/ERK | + | + | |||||||
| RhoA/ROCK | + | ||||||||
| Rho GTPase | + | ||||||||
| TGF-β | + | + | + | + | + | ||||
| TGF-β/Smad | + | + | + | ||||||
| Wnt/β-catenin | + | + | + | + | + | + | + | + |
7. ECM in Solid Cancers
Tumour progression requires continuous interaction between the ECM and tumour cells where increased cytoskeleton signalling reduces the number of intercellular adhesion molecules, induces mesenchymal transition, upregulates membrane integrins, enhances the migratory potential [7] and therapeutic resistance [67]. Escalated deposition and cross-linking of collagen interfere with cell polarity, cell adhesion, and integrin signalling and promote tumour progression. ECM degradation and loss of BM integrity due to MMP are hallmarks of invasive lesions [79]. Another cancer hallmark is the tumour cell production of glycoproteins and proteoglycans with altered glycosylation (e.g., cancer antigen 19-9 and 125, carcinoembryonic antigen, prostate-specific antigen and alpha-fetoprotein) secreted or shed from the cell membranes into the bloodstream where they may serve as tumour-associated biomarkers) [80]. ECM—the largest part of a solid tumour—applies mechanical and non-mechanical forces on tumour tissue, such as stiffening, increased interstitial fluid pressure, collapsing vessels, hypoxia, and acidity [81] and compromises the outcome of oncological treatments and prognosis [82]. In melanoma and breast cancer, increased amounts of collagen I correlated with disease progression and reduced survival [83,84,85][83][84][85]. Further, collagen and fibronectin are involved in angiogenesis. Interacting with α1β1, α2β1, ανβ3, and ανβ5 integrins, collagen I activates mitogen-activated protein kinase (MAPK) pathways supporting the survival of endothelial cells, remodelling the actin cytoskeleton, and influencing the cells to form lumens [86]. Fibronectin plays a pivotal role in the assembly of vascular matrix components [87], while MMP releases GF, VEGF, and chemokines bound within the ECM [88,89][88][89] to remodel the ECM and direct endothelial migration and capillary movements along aligned ECM fibres [90,91][90][91]. When nutrient levels run low, integrin-bound glycoproteins of the ECM are easily endocytosed and internalised into lysosomes by tumour cells. The role of ECM as a nutrient provider represents a potential therapeutic target to inhibit integrin uptake in stromal-enriched cancers [92]. Ligand-bound integrin trafficking has recently been shown to affect nutrient signalling through the rapamycin (mTOR) signalling pathway [92,93][92][93]. With sufficient nutrient availability, mTOR induces anabolic processes such as protein, nucleotide, and lipid biosynthesis and inhibits lysosomal biogenesis and cellular autophagy [94] through two independent complexes of mTOR. The complex mTORC1 adjusts cell growth and proliferation in response to GF and amino acids, while mTORC2 is involved in actin organisation and cell proliferation and survival [95]. During nutrient starvation, the activity of mTORC1 is downregulated, allowing cells to use other sources of nutrient acquisition, such as autophagy [96,97][96][97]. The ECM-attached cells induce adaptive responses or compensatory homeostatic feedback loops, leading to the induction of several pro-survival proteins, including receptor tyrosine kinases and antiapoptotic proteins including the activation of MAPK, PI3K, AKT and the human epidermal growth factor 3 (HER3) [98]. The alignment of collagen fibres by lysyl oxidase (LOX) produced by cancer cells and CAF directs cancer cell migration and induces their proliferation [99,100,101][99][100][101]. In an in vitro study on benign and malignant human ovarian cell lines, the ability to rapidly remodel the matrix enabled tumour cell migration along aligned fibres and changed direction according to microenvironmental cues [102]. The ECM is a storage facility for nutrients, proteases, morphogens, and GF that create pro-migratory gradients. These molecules are released by proteolytic degradation of the ECM, which regulates the rate and intensity of migration [103]. Due to the ongoing modulation of the ECM, the TME is rich in protease-digested fragments that may influence the metastatic potential and apoptosis of cancer cells [104]. These fragments may even display opposite effects as compared to their molecules of origin, making studies on the TME even more challenging [105]. Notably, the binding of an ECM-associated GF to its receptor (GFR) may prevent its endocytosis and secure prolonged, upregulated signalling by the ECM-GF/GFR complex [106]. In addition, cells may generate different responses to the same effector molecule bound to different matrix molecules under the same GF conditions [107].References
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell. Signal. 2021, 85, 110046.
- Li, W.; Chi, N.; Rathnayake, R.A.C.; Wang, R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: A nanoscopic study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71.
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914.
- Song, D.; Shivers, J.L.; MacKintosh, F.C.; Patteson, A.E.; Janmey, P.A. Cell-induced confinement effects in soft tissue mechanics. J. Appl. Phys. 2021, 129, 140901.
- Müller, P.; Rogers, K.W.; Yu, S.R.; Brand, M.; Schier, A.F. Morphogen transport. Development 2013, 140, 1621–1638.
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492.
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120.
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599, 673–678.
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063.
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. Methods Mol. Biol. 2021, 2347, 17–25.
- Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P. Collagen Structure-Function Mapping Informs Applications for Re-generative Medicine. Bioengineering 2020, 8, 3.
- Hsu, K.-S.; Dunleavey, J.M.; Szot, C.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Feng, Y.; Lutz, E.M.; Koogle, R.; et al. Cancer cell survival depends on collagen uptake into tumor-associated stroma. Nat. Commun. 2022, 13, 7078.
- Ma, W.; Ma, H.; Fogerty, F.J.; Mosher, D.F. Bivalent ligation of the collagen-binding modules of fibronectin by SFS, a non-anchored bacterial protein of streptococcus equi. J. Biol. Chem. 2015, 290, 4866–4876.
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747.
- Burke, L.; Guterman, I.; Gallego, R.P.; Britton, R.G.; Burschowsky, D.; Tufarelli, C.; Rufini, A. The Janus-like role of proline metabolism in cancer. Cell Death Discov. 2020, 6, 104.
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329.
- Kuivaniemi, H.; Tromp, G.; Prockop, D. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum. Mutat. 1999, 9, 300–331. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1098-1004(1997)9:4%3C300::AID-HUMU2%3E3.0.CO;2-9 (accessed on 13 February 2023).
- Hwang, S.J.; Ha, G.H.; Seo, W.Y.; Kim, C.K.; Kim, K.J.; Lee, S.B. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep. 2020, 53, 539–544.
- Wanjare, M.; Agarwal, N.; Gerecht, S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Physiol. 2015, 309, C271–C281.
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205.
- Schmelzer, C.E.H.; Heinz, A.; Troilo, H.; Lockhart-Cairns, M.P.; Jowitt, T.A.; Marchand, M.F.; Bidault, L.; Bignon, M.; Hedtke, T.; Barret, A.; et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019, 33, 5468–5481.
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273.
- Shapiro, S.D.; Endicott, S.K.; Province, M.; Pierce, J.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834.
- Tembely, D.; Henry, A.; Vanalderwiert, L.; Toussaint, K.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Jaisson, S.; Galés, C.; Martiny, L.; et al. The Elastin Receptor Complex: An Emerging Therapeutic Target Against Age-Related Vascular Diseases. Front. Endocrinol. 2022, 13, 136.
- Thorlacius-Ussing, J.; Kehlet, S.N.; Rønnow, S.R.; Karsdal, M.A.; Willumsen, N. Non-invasive profiling of protease-specific elastin turnover in lung cancer: Biomarker potential. J. Cancer Res. Clin. Oncol. 2018, 145, 383–392.
- Li, J.; Xu, X.; Jiang, Y.; Hansbro, N.G.; Hansbro, P.M.; Xu, J.; Liu, G. Elastin is a key factor of tumour development in colorectal cancer. BMC Cancer 2020, 20, 217.
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; van Obberghen-Schilling, E. Shaping Up the Tumour Microenvironment with Cellular Fibronectin. Front. Oncol. 2020, 10, 641.
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2014, 34, 3315–3324.
- Patankar, M.; Eskelinen, S.; Tuomisto, A.; Karttunen, T.J. KRAS and BRAF mutations induce anoikis resistance and characteristic 3D phenotypes in Caco-2 cells. Mol. Med. Rep. 2019, 20, 4634–4644.
- Yu, Y.; Liu, B.; Li, X.; Lu, D.; Yang, L.; Chen, L.; Li, Y.; Cheng, L.; Lv, F.; Zhang, P.; et al. ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell Death Dis. 2022, 13, 46.
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-κB. Oncogene 2006, 25, 4341–4349.
- Gee, E.P.S.; Yüksel, D.; Stultz, C.M.; Ingber, D.E. SLLISWD sequence in the 10FNIII domain initiates fibronectin fibrillogenesis. J. Biol. Chem. 2013, 288, 21329–21340.
- Ohashi, T.; Kiehart, D.P.; Erickson, H.P. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA 1999, 96, 2153–2158.
- Paten, J.A.; Martin, C.L.; Wanis, J.T.; Siadat, S.M.; Figueroa-Navedo, A.M.; Ruberti, J.W.; Deravi, L.F. Molecular Interactions between Collagen and Fibronectin: A Reciprocal Relationship that Regulates De Novo Fibrillogenesis. Chem 2019, 5, 2126–2145.
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028.
- Griggs, L.A.; Hassan, N.T.; Malik, R.S.; Griffin, B.P.; Martinez, B.A.; Elmore, L.W.; Lemmon, C.A. Fibronectin fibrils regulate TGF-β1-induced Epithelial-Mesenchymal Transition. Matrix Biol. 2017, 60–61, 157–175.
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumour aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum. Pathol. 2013, 44, 2028–2037.
- Wang, J.; Li, R.; Li, M.; Wang, C. Fibronectin and colorectal cancer: Signaling pathways and clinical implications. J. Recept. Signal Transduct. 2020, 41, 313–320.
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191.
- Joseph, J.V.; Conroy, S.; Pavlov, K.; Sontakke, P.; Tomar, T.; Eggens-Meijer, E.; Balasubramaniyan, V.; Wagemakers, M.; Dunnen, W.F.D.; Kruyt, F.A. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α–ZEB1 axis. Cancer Lett. 2015, 359, 107–116.
- Ryu, M.H.; Park, H.M.; Chung, J.; Lee, C.H.; Park, H.R. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma in-vasion via upregulation of alpha5 integrin and fibronectin. Biochem. Biophys. Res. Commun. 2010, 393, 11–15.
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250–263.
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587.
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018, 75–76, 12–26.
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163.
- Islam, K.; Thummarati, P.; Kaewkong, P.; Sripa, B.; Suthiphongchai, T. Role of laminin and cognate receptors in cholangiocarcinoma cell migration. Cell Adhes. Migr. 2021, 15, 152–165.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55.
- Gopal, S.; Amran, A.; Elton, A.; Ng, L.; Pocock, R. A somatic proteoglycan controls Notch-directed germ cell fate. Nat. Commun. 2021, 12, 6708.
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat. Res. Commun. 2021, 27, 100312.
- Scott, J. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645.
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606.
- Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C. Molecular Cell Biology, 5th ed.; W.H Freeman: New York, NY, USA, 2003.
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525.
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18.
- Enemark, M.B.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.; d’Amore, F.; Ludvigsen, M. Tumour-Tissue Expression of the Hyaluronic Acid Receptor RHAMM Predicts Histological Transformation in Follicular Lymphoma Patients. Cancers 2022, 14, 1316.
- Auvinen, P.; Tammi, R.; Kosma, V.-M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2012, 132, 531–539.
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int. J. Mol. Sci. 2022, 24, 103.
- Kim, P.K.; Halbrook, C.J.; Kerk, S.A.; Radyk, M.; Wisner, S.; Kremer, D.M.; Sajjakulnukit, P.; Andren, A.; Hou, S.W.; Trivedi, A.; et al. Hyaluronic acid fuels pancreatic cancer cell growth. eLife 2021, 10, e62645.
- Zhang, G.; Guo, L.; Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Gao, F. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumour-associated macrophages formation. Oncoimmunology 2016, 5, e1172154.
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumour-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595.
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients: A protocol for systematic review and meta analysis. Medicine 2020, 99, e20438.
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immuno-therapy. Nat. Biotechnol. 2020, 38, 947–953.
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. Ebiomedicine 2022, 76, 103873.
- Chen, X.; Zhu, H.; Feng, X.Q.; Li, X.; Lu, Y.; Wang, Z.; Rezgui, Y. Predictive assembling model reveals the self-adaptive elastic properties of lamellipodial actin networks for cell migration. Commun. Biol. 2020, 3, 616.
- Grandy, C.; Port, F.; Pfeil, J.; Gottschalk, K.-E. Influence of ROCK Pathway Manipulation on the Actin Cytoskeleton Height. Cells 2022, 11, 430.
- Northey, J.J.; Przybyla, L.; Weaver, V.M. Tissue force programs cell fate and tumour aggression. Cancer Discov. 2017, 7, 1224–1237.
- Han, S.J.; Azarova, E.V.; Whitewood, A.J.; Bachir, A.; Guttierrez, E.; Groisman, A.; Horwitz, A.R.; Goult, B.T.; Dean, K.M.; Danuser, G.; et al. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. eLife 2021, 10, e66151.
- Seetharaman, S.; Vianay, B.; Roca, V.; Farrugia, A.J.; De Pascalis, C.; Boëda, B.; Dingli, F.; Loew, D.; Vassilopoulos, S.; Bershadsky, A.; et al. Microtubules tune mechanosensitive cell responses. Nat. Mater. 2021, 21, 366–377.
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82.
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905.
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133, jcs230425.
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53.
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2021, 21, 60–78.
- Zhang, P.; Wang, J.; Luo, W.; Yuan, J.; Cui, C.; Guo, L.; Wu, C. Kindlin-2 Acts as a Key Mediator of Lung Fibroblast Activation and Pulmonary Fibrosis Progression. Am. J. Respir. Cell Mol. Biol. 2021, 65, 54–69.
- Guo, L.; Cui, C.; Zhang, K.; Wang, J.; Wang, Y.; Lu, Y.; Chen, K.; Yuan, J.; Xiao, G.; Tang, B.; et al. Kindlin-2 links mechano-environment to proline synthesis and tumour growth. Nat. Commun. 2019, 10, 845.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumour Microenvironment. Cell 2010, 141, 52–67.
- Silsirivanit, A. Chapter Five: Glycosylation markers in cancer. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 189–213.
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Tumour Vasculature: Improving Drug Delivery and Efficacy. Trends Cancer 2018, 4, 258–259.
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238.
- Miskolczi, Z.; Smith, M.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene 2018, 37, 3166–3182.
- Ajeti, V.; Nadiarnykh, O.; Ponik, S.M.; Keely, P.J.; Eliceiri, K.W.; Campagnola, P.J. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: Implications for probing stromal alterations in human breast cancer. Biomed. Opt. Express 2011, 2, 2307–2316.
- Kauppila, S.; Stenbäck, F.; Risteli, J.; Jukkola, A.; Risteli, L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1999, 186, 262–268.
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456.
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin Deposition Participates in Extracellular Matrix Assembly and Vascular Morphogenesis. PLoS ONE 2016, 11, e0147600.
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.S.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumour microenvironment: Colon tumour-derived extracellular matrix promotes angiogenesis and tumour cell growth. Biomaterials 2017, 116, 118–129.
- Ruehle, M.A.; Eastburn, E.A.; LaBelle, S.A.; Krishnan, L.; Weiss, J.A.; Boerckel, J.D.; Wood, L.B.; Guldberg, R.E.; Willett, N.J. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 2020, 6, eabb6351. Available online: https://www.science.org/doi/10.1126/sciadv.abb6351 (accessed on 21 November 2022).
- Daub, J.T.; Merks, R.M.H. A Cell-Based Model of Extracellular-Matrix-Guided Endothelial Cell Migration During Angiogenesis. Bull. Math. Biol. 2013, 75, 1377–1399.
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Muranen, T.; Iwanicki, M.P.; Curry, N.L.; Hwang, J.; DuBois, C.D.; Coloff, J.L.; Hitchcock, D.S.; Clish, C.B.; Brugge, J.S.; Kalaany, N.Y. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017, 8, 13989.
- Rainero, E.; Howe, J.D.; Caswell, P.T.; Jamieson, N.B.; Anderson, K.; Critchley, D.R.; Machesky, L.; Norman, J.C. Ligand-Occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015, 10, 398–413.
- Kim, S.G.; Hoffman, G.R.; Poulogiannis, G.; Buel, G.R.; Jang, Y.J.; Lee, K.W.; Kim, B.Y.; Erikson, R.L.; Cantley, L.C.; Choo, A.Y.; et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 2012, 49, 172–185.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976.
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumour Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239.
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460.
- Muranen, T.; Selfors, L.M.; Worster, D.T.; Iwanicki, M.P.; Song, L.; Morales, F.C.; Gao, S.; Mills, G.B.; Brugge, J.S. Inhibition of PI3K/mTOR Leads to Adaptive Resistance in Matrix-Attached Cancer Cells. Cancer Cell 2012, 21, 227–239.
- Koorman, T.; Jansen, K.A.; Khalil, A.; Haughton, P.D.; Visser, D.; Rätze, M.A.K.; Haakma, W.E.; Sakalauskaitè, G.; van Diest, P.J.; de Rooij, J.; et al. Spatial collagen stiffening promotes collective breast cancer cell invasion by reinforcing extracellular matrix alignment. Oncogene 2022, 41, 2458–2469.
- Esfahani, P.; Levine, H.; Mukherjee, M.; Sun, B. Three-dimensional cancer cell migration directed by dual mechanochemical guidance. Phys. Rev. Res. 2022, 4, L022007.
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Liu, R.; Qu, J. Oriented collagen fibers direct tumour cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213.
- Piotrowski-Daspit, A.S.; Nerger, B.A.; Wolf, A.E.; Sundaresan, S.; Nelson, C.M. Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophys. J. 2017, 113, 702–713.
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS ONE 2020, 15, e0220019.
- Gaggar, A.; Weathington, N. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Investig. 2016, 126, 3176–3184.
- He, X.; Lee, B.; Jiang, Y. Extracellular matrix in cancer progression and therapy. Med. Rev. 2022, 2, 125–139.
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219.
- Olabi, S.; Ucar, A.; Brennan, K.; Streuli, C.H. Integrin-Rac signalling for mammary epithelial stem cell self-renewal 06 Biological Sciences 0601 Biochemistry and Cell Biology. Breast Cancer Res. 2018, 20, 128.
- Wei, S.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.; et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688.
- Barrera, L.N.; Ridley, P.M.; Bermejo-Rodriguez, C.; Costello, E.; Perez-Mancera, P.A. The role of microRNAs in the modulation of cancer-associated fibroblasts activity during pancreatic cancer pathogenesis. J. Physiol. Biochem. 2022, 79, 193–204.
More
