Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Robert Aurelian Tiuca and Version 4 by Dean Liu.
Thyroid cancer is the most common endocrine malignancy, with an increasing trend in the past decades. It has a variety of different histological subtypes, the most frequent one being differentiated thyroid cancer, which refers to papillary carcinoma, the most common histological type, followed by follicular carcinoma. Associations between genetic polymorphisms and thyroid cancer have been investigated over the years and are an intriguing topic for the scientific world.
- thyroid cancer
- differentiated thyroid cancer
- thyroid cancer prognosis
- molecular biomarkers
- targeted therapy
- single nucleotide polymorphism
- genetic variation
- genetic predisposition
1. Introduction
Thyroid cancer, the most frequent endocrine malignancy, has recorded an increase in global incidence in recent decades. This increase is mostly attributed to the increased detection of low-risk tumors through intensive screening, which may result in an overdiagnosing phenomenon [1]. Papillary thyroid carcinoma (PTC) is the most common histological type, followed by follicular thyroid carcinoma (FTC). The papillary and follicular histological types are known as differentiated thyroid cancer (DTC) to distinguish them from poorly differentiated thyroid and anaplastic carcinomas, which originate from the follicular tissue, as well as from medullary thyroid cancer, which is derived from the parafollicular C cells [2]. Several risk factors, modifiable and non-modifiable, have been linked to an increased likelihood of developing thyroid cancer (Figure 1) [3].
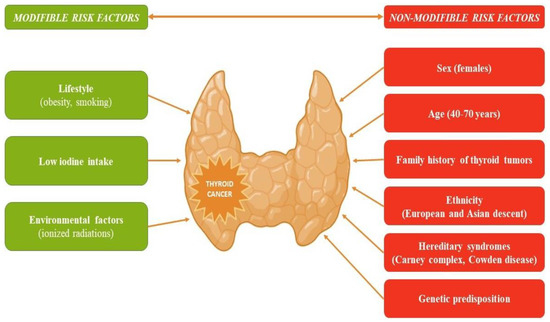
Figure 1.
Risk factors that increase the likelihood of developing thyroid cancer.
The prognosis in DTC is often favorable, but it is influenced by the tumor’s histological type. PTC has an excellent prognosis, although a small proportion of patients show an aggressive, unfavorable evolution [4][5][4,5]. In recent years, molecular markers such as B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF) V600E and Neuroblastoma RAS viral oncogene homolog (NRAS) mutations, and more recently, mutations in telomerase reverse transcriptase promoter (pTERT), have been studied for their role as predictors in treatment response, as well as their association with various clinico-pathological features, recurrence, and mortality in patients with DTC [6][7][6,7]. The co-existence of pTERT and the BRAF V600E mutation is associated with an unfavorable prognosis, distant metastases, and increased mortality [8][9][8,9]. As opposed to genetic mutation, which is defined as any abnormal change in a DNA sequence, a genetic polymorphism is a DNA sequence variation commonly found in the population [10].
The identification of patients diagnosed with thyroid cancer at risk of having an unfavorable prognosis has great importance in optimizing individual therapeutic management. ReThisearchers aim narrative review aims to emphasize the existing literature data regarding genetic polymorphisms investigated for their potential association with DTC and highlight the opportunity of using genetic variations as biomarkers of diagnosis and prognosis for thyroid cancer patients.
2. SNPs of Tyrosine-Kinase Family Genes
2.1. SNPs of RET Gene
The Rearranged during Transfection (RET) protooncogene encodes a receptor of the tyrosine-kinase proteins and has been associated with numerous types of cancers, including thyroid cancer. The process leading to carcinogenesis implies gain-of-function mutations that translate to RET activation [11][22]. Santos et al. conducted a case-control study in 2014 on a total of 1088 individuals (545 with DTC and 543 controls) to determine the effect of four SNPs (G691S, L769L, S836S, and S904S) of RET in the risk for DTC. It was noted that RET S836S is overexpressed in patients with PTC as well as the GGTC haplotype, suggesting that it might be a risk factor for PTC. Interestingly, the overrepresentation of minor alleles G691S and S904S was identified in an almost significant percentage of tumors >10 mm at diagnosis, hinting at a possible role in tumor behavior. No such associations were found in FTC [12][23]. On the other hand, He et al. noted that the haplotype CGGATAA of rs17028, rs1799939, rs1800858, rs1800860, rs2075912, rs2565200, and rs2742240 was associated with a reduced risk of DTC (OR = 0.18, p = 0.001) in a candidate gene association study conducted on 552 subjects (300 with DTC and 252 controls). In the same study, rs1799939 AG or AG plus AA genotypes were associated with increased risk for DTC without concomitant thyroid benign disorders (OR = 1.93, p = 0.009 and OR = 1.88, p = 0.011, respectively). Moreover, an increased risk for distant metastases was found for haplotype CAAGCGT of rs17028, rs1799939, rs1800858, rs1800860, rs2075912, rs2565200, and rs2742240 (OR = 7.57, p = 0.009) [13][24]. Although this research offers interesting information, the study population involved in both studies was relatively small, therefore the impact of RET SNPs on DTC risk might be underestimated. Furthermore, neither one of the studies assessed radiation exposure, a factor that has a great impact on the oncogenesis of thyroid malignancy. A recent meta-analysis showed strong epidemiological evidence of associations through the Venice criteria and false-positive report probability between thyroid cancer susceptibility and RET rs1799939 [14][25].2.2. SNPs of MET Gene
The cellular mesenchymal-epithelial transition (MET) factor is a plasma membrane tyrosine kinase receptor with low activity in normal cells, which may become activated in tumor cells through mutations, amplifications, or overexpression, leading to a potential increase in the aggressiveness of cancer [15][16][26,27]. The role of MET gene SNPs in PTC was investigated by Ning et al., who evaluated 858 patients with PTC [17][28]. MET SNP (rs1621) showed a significant association with PTC in females. Moreover, the rs1621 AG genotype might be especially associated with the female sex as it was significantly higher in the PTC group for female patients and had an increased risk of PTC. On the other hand, the analyzed MET SNPs revealed no correlation with metastasis and prognosis, regardless of gender [17][28].3. SNPs of Genes Involved in Apoptosis, Genome Stability, and DNA Repair
3.1. SNPs of BAX Gene
B-cell lymphoma 2-associated X protein (BAX) has an important role in mitochondrial apoptosis [18][29]. There might be an association between BAX gene polymorphism and oncogenesis, mainly due to the −248 G > A polymorphism that down-regulates the BAX gene transcription which ultimately might inhibit the apoptosis in tumoral cells [19][30]. However, a meta-analysis from 2013 suggested that BAX −248 G > A polymorphism is not to be considered an important oncogenic factor [20][31]. As for research which assessed this polymorphism and DTC, Cardoso-Duarte et al. recently conducted a case-control study on 30 Brazilian patients with PTC and concluded that the BAX single nucleotide polymorphism −248 G > A GG genotype was associated with PTC and the presence of the G allele was a protective factor against the occurrence of PTC [21][32].3.2. SNPs of TP53 and p21 Gene
It is well known that events leading to DNA damage critically raise the risk of developing cancer. Tumor protein 53 (TP53) has a main role in protecting the genome from getting damaged; therefore, it plays an important part in cancer development, including thyroid cancer [22][33]. Depending on the severity of DNA damage, TP53 determines if the cell undergoes a repairing process or apoptosis [23][34]. The p21 protein is a cyclin-dependent kinase inhibitor that is expressed by the activated TP53 during the G1 phase of the cell cycle, inhibiting DNA replication and therefore stopping the progression of the cell cycle [24][35]. Therefore, there are data stating that in more than half of all cancers, TP53/p21 is inactivated [25][36]. Heidari et al. investigated if SNPs in TP53 (rs1042522) and p21 (rs1059234 and rs1801270) genes affect the risk of PTC or if they are associated with clinical and histopathological features of PTC. The study was designed as a case-control study and found that the TP53-rs1042522 CC genotype was significantly associated with protection against PTC in the dominant, recessive, and allelic models (p = 0.008, p = 0.01, p = 0.002, respectively). Moreover, the rs1042522 was associated with tumors > 1 cm in dominant and recessive models (p = 0.04, p = 0.009, respectively) and with vascular invasion in the dominant model (p = 0.01). However, regarding SNP of the p21 gene (rs10559234 and rs1801270), no correlation was found with the risk of PTC or clinical and histopathologic features [26][37]. There is little data in the literature concerning SNP in these genes and their role in thyroid cancer; therefore, there is a need to expand the research towards this area knowing the role they have in suppressing carcinogenesis.3.3. SNPs of HOTAIR Gene
SNPs in the HOX Transcript Antisense RNA (HOTAIR) gene, which is an oncogene that regulates gene expression and chromatin dynamics, have been associated with breast and colorectal cancers [27][28][38,39]. Regarding thyroid cancer, there are mixed results depending on the SNP studied. Rad et al. found that HOTAIR rs1899663 gene polymorphism was not associated with clinical or histopathological features of thyroid cancer [29][40]. On the other hand, Min et al. showed that HOTAIR rs12826786 and rs920778 had an increased thyroid cancer risk, whereas rs7958904, rs4759314, rs874945, and rs189963 did not correlate with increased thyroid cancer risk [30][41].3.4. SNPs of XRCC1 Gene
The X-ray repair cross-complementing group 1 (XRCC1) proteins have a central role in the base excision repair, an essential DNA repair pathway, thus preserving the genome stability [31][42]. In the past years, the role of the XRCC1 Arg194Trp polymorphism in the development of DTC has been investigated. A meta-analysis published in 2016 by Zhao JZ et al. pointed out the XRCC1 Arg194Trp polymorphism had an increased thyroid cancer risk in the Caucasian population [32][43]. On the other hand, in a more recent study published by Liu SY that also investigated XRCC1 Arg194Trp polymorphism and its association with susceptibility to thyroid cancer, the C allele of XRCC1 had an 18% significantly decreased risk of thyroid cancer in Chinese people, but without any association among Caucasians [33][44].4. SNPs of the VDR Gene
Low vitamin D levels were correlated with an increased risk of advanced papillary thyroid cancer in several studies, being associated with local or distant metastasis and having a potential prognostic impact [34][35][36][45,46,47]. Vitamin D receptor (VDR) polymorphisms might increase the risk of several types of cancer (breast, ovarian, colorectal) [37][38][39][48,49,50]. Regarding thyroid cancer, Beysel et al. published a case-control study in 2018 and found that the VDR gene FokI (rs2228570) CT/TT genotype had an increased risk of PTC, whereas the FokI TT genotype usually presented with increased tumor diameter (T3 and T4), advanced stage (III/IV), and extra-thyroidal invasion. Moreover, the FokI CT/TT or TT genotypes were associated with lymph node metastasis, multifocality, and tumors >10 mm [40][51]. Another study conducted on Romanian patients with DTC investigated the correlation between vitamin D levels, VDR gene polymorphisms, clinical findings, and histopathological traits and found that vitamin D levels were significantly lower in patients with DTC and that FokI polymorphisms were frequently encountered in patients with DTC. Moreover, the Ff genotype was associated with more aggressive forms [41][52]. These studies showed promising results and future research should focus on studying in a more detailed manner the opportunities of using FokI as a poor prognostic factor in DTC.5. SNPs of Extracellular Matrix Genes with Roles in Cellular Proliferation and Differentiation
5.1. SNPs of SPARC and SPP1 Gene
Matricellular proteins such as secreted phosphoprotein 1 (SPP1) and secreted protein acidic and rich in cysteine (SPARC) are involved in preserving cellular stability and structure [42][53]. The aberrant expression of these proteins might lead to several types of cancer progression or metastases [43][44][54,55]. Su X et al. noted that three loci, rs1054204, rs3210714, and rs3549 in SPARC and rs4754 in SPP1, individually or combined, are involved in PTC susceptibility, either decreasing or increasing the risk of developing the disease [45][56]. Currently, data regarding genetic variants of matricellular proteins in thyroid cancer are scarce, therefore there is great potential to explore this research area through future studies.
5.2. SNPs of MMP-9 Gene
Matrix metalloproteinases, also knowns as matrixins, are calcium-dependent and zinc-containing endoproteinases, which are capable of degrading numerous extracellular matrix proteins and processing several types of bioactive molecules, having an important part in processes such as cell proliferation, adhesion/dispersion, angiogenesis, and apoptosis [46][57]. Matrix metalloproteinase-9 (MMP-9) is one of the most important and complex MMPs, with implications in inflammatory activity, immune response, activation of tumor growth factor β in cancer progression, and resistance of tumor cells, which has a strong application as a cancer growth, invasion, and metastasis mediator [47][58]. MMP-9 has a physiologically low-level expression but is overexpressed in several types of cancers [47][48][58,59].
Serum MMP-9 levels measured using an immunometric assay were evaluated in subjects with PTC in a case-control study that concluded that although serum MMP-9 is not helpful in the diagnosis of PTC, a high pre-surgical level might be a prognostic factor that could dictate the treatment aggressiveness [49][60]. In a recent study that investigated the role of MMP-9 promoter −1562C/T functional SNP in the risk of developing PTC, the T allele was found significantly more frequently in subjects with PTC (17.5% vs. 10.1%, p = 0.019) [50][61]. PTC had an increased risk of developing in subjects with the CT or CT + TT genotype. Although MMP-9 promoter SNP seems to increase the susceptibility of developing PTC, it was not associated with tumor histological subtype, invasion, or tumor stage [50][61]. Nevertheless, despite these promising results that strongly suggest a role for MMP-9 SNPs in thyroid carcinogenesis, future research is still needed on larger populations.
5.3. SNPs of NRG1 Gene
The neuregulin 1 (NRG1) gene encodes a glycoprotein that mediates cell–cell signaling. It is produced in multiple isoforms and plays an important part in the proliferation, survival, and differentiation of various system organs [51][62]. Several studies demonstrated an association between NRG1 rs2439302 and PTC [51][52][53][62,63,64]. Moreover, a recent meta-analysis consolidated the previous results stating that the SNP rs2439302 variants of the gene encoding NRG1 carry a high thyroid cancer risk, especially in Chinese populations compared to Japanese populations or populations from the United States of America [54][65].
