Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Joaquin L. Herraiz.
Ultrasound (US) is a flexible imaging modality used globally as a first-line medical exam procedure in many different clinical cases. It benefits from the continued evolution of ultrasonic technologies and a well-established US-based digital health system. Nevertheless, its diagnostic performance still presents challenges due to the inherent characteristics of US imaging, such as manual operation and significant operator dependence. Artificial intelligence (AI) has proven to recognize complicated scan patterns and provide quantitative assessments for imaging data.
- ultrasound
- computer-assisted ultrasonography
- neural network
- deep learning
1. Introduction
The connection between medicine and computer science started in the early 1960s, when scientists developed a digital computer-based monitoring system [1] that laid the foundations of the current standard in intensive care units across the world. Since then, the relationship between clinical practice and computing advances has been constant for the last half century [2]. Computer capabilities have been evolving at an exponential rate thanks to Moore’s Law and significant improvements in processor design, and now the recent developments in artificial intelligence (AI) and deep learning (DL) methods are starting to fulfill some of the promises of computer science pioneers [3].
Software systems in medicine have taken advantage of the increasing capabilities of modern computers to provide real-time solutions to intricate challenges. Complex medical imaging problems, such as computer-assisted ultrasonography, are now being tackled with AI with great results by exploiting the collaborative work of physicians and engineers, the capabilities of modern computing hardware [4[4][5],5], and the access to digital medical images.
Nevertheless, the introduction of deep learning-based strategies into clinical practice has just started, and much work lies ahead. Many leading AI experts agree that there is a huge gap between proof-of-concept research papers and actually deploying a machine learning (ML) system in the real world [6], especially in clinical devices that have to pass regulatory processes. In fact, there is undoubtedly huge potential for ML to transform healthcare, but going ‘from code to clinic’ is the hardest part. The list of challenges to overcome in this field [6] includes the difficulties in obtaining datasets with sufficiently large, curated, high-quality data; the lack of robust clinical validations; the need for interdisciplinary collaborations of computational scientists, signal processing engineers, physics experts, and medical experts [7]; and some technical limitations of AI algorithms.
2. Current Challenges of Ultrasound Imaging
Portable ultrasound has the potential to become the new stethoscope. The ultrasound imaging modality stands out for its safety, portability, non-invasiveness, and relatively low cost, which support its use in many different clinical scenarios. In fact, ultrasound imaging has become an important diagnostic tool for an increasing number and range of clinical conditions, enabling a greater variety of treatment options. This situation has led to the design of multiple complex diagnostic procedures and the atomization of their medical use.
However, there are several challenges currently associated with ultrasound imaging:
-
Operator dependency. As US imaging depends on the ability of the operator to position the transducer and interpret the images correctly, it can be difficult to obtain consistent and reliable results from different operators, requiring long and specialized training.
-
Subjectivity in image interpretation. The interpretation of the US images is quite subjective. Unlike other imaging modalities, which produce objective, quantitative data, the interpretation of US images depends heavily on the experience and skills of the person performing the scan. This can lead to variability in the accuracy and reliability of the images, particularly when they are used for diagnostic purposes.
-
Limited penetration depth, which limits its use for imaging deep structures in the body, and an inability to penetrate certain tissues or structures, such as bone and air-filled cavities.
-
Limited quality of US images. Ultrasound images are typically less detailed than those produced by other modalities. This can make it difficult to accurately diagnose certain conditions, particularly when the structures being imaged are small or located deep within the body.
While advances in technology are helping to address some of these challenges, they still remain an important consideration in the use of ultrasound imaging.
3. The Deep-Learning Revolution in Ultrasound Imaging
The use of deep learning in medical imaging is one of the fields that has reached the most significant advances in the field of AI [8]. It has already achieved many meaningful results in US imaging [9,10][9][10]. The tremendous potential of the combination of both clinical and commercial technologies is widely recognized, making computer-assisted ultrasound a hot research field.
As shown in Figure 1, the number of academic publications on “deep learning” in Pubmed has soared from less than 1000 in 2017 to more than 16,000 in 2022, and the number of deep learning applications in ultrasound imaging has also followed the same exponential trend. Furthermore, if we consider that, despite their high impact and consideration within the community, conference proceedings are usually not listed under PubMed and other databases may include some additional publications not included in PubMed, the number of recent scientific works on ultrasound and artificial intelligence is even higher than shown in Figure 1. These numbers demonstrate the current enthusiasm for this new technology.
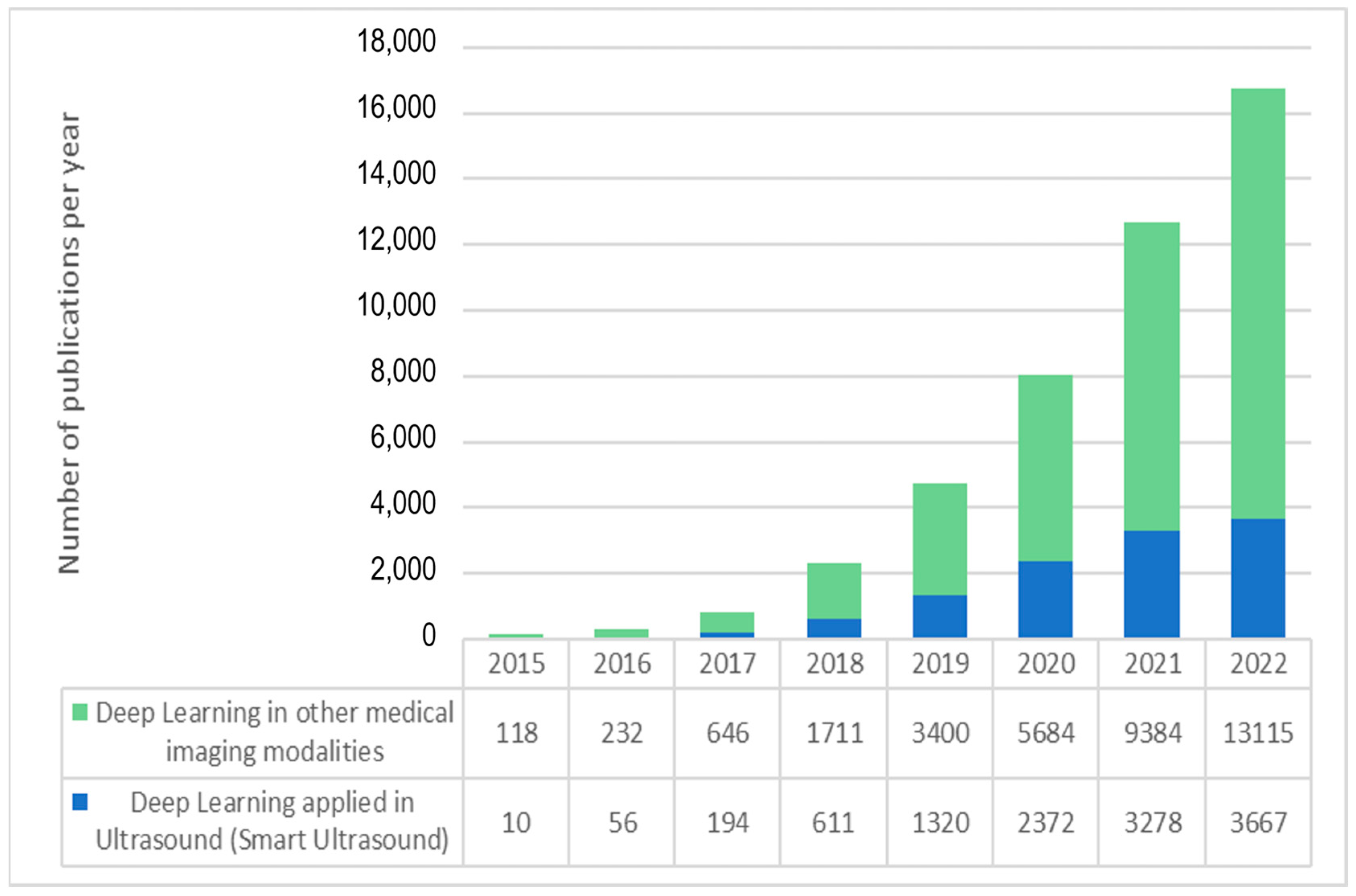
Figure 1. Exponential growth in the number of articles indexed in PubMed with “deep learning” and “ultrasound” (blue) and “deep learning” applied to other data and medical image modalities.
4. Main Applications of AI in US Imaging
Figure 2 shows a diagram of the diagnostic workflow with computer-assisted US devices, where the main tasks performed during the examination are outlined. Some of them are done by practitioners (and technicians), others are in charge of the hardware, but a significant and growing portion of them are evolving as advanced software pieces that try to improve the efficiency of the two previous actors. The illustrated workflow starts by placing the probe on the patient’s body surface, which will be traversed by pressure waves whose echo is accurately measured. US imaging systems process the recorded signals to generate 2D images, which are taken to perform measurements and diagnoses. Assistance can be provided in the examination procedure by providing indications on how to perform the scan, recognizing the scanned organ, and making additional assessments about how to improve the image quality in the acquired frames.
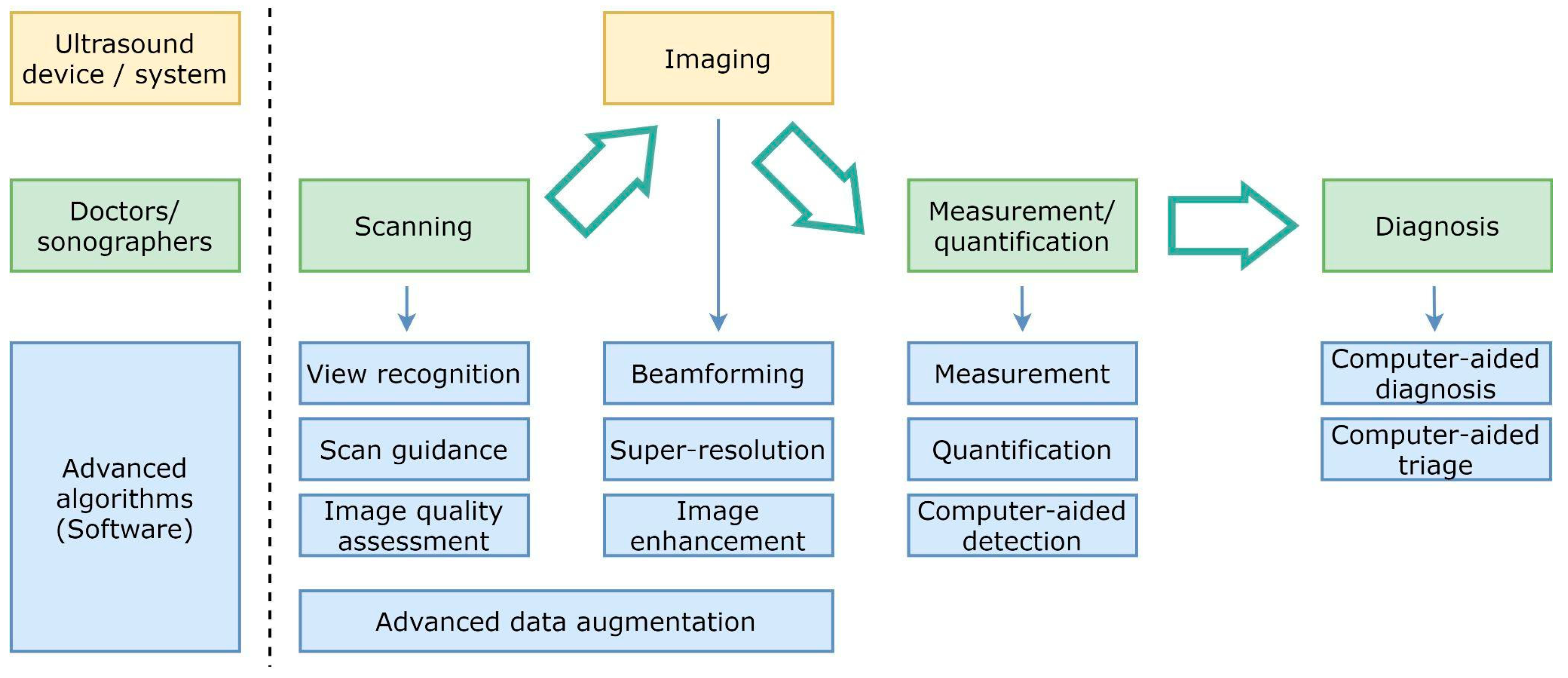
Figure 2.
Simplified diagram of the diagnostic workflow with computer-assisted ultrasound devices.
Beamforming, higher resolution, and image enhancement are three areas in which conventional signal processing can be improved. Computer assistance tools could also replace repetitive measurement and quantification tasks. Additionally, physicians will be able to consider additional feedback from computer-aided diagnosis and computer-aided triage tools.
There are four main research areas where artificial intelligence and computer-assisted solutions may help standardize medical practice, reduce training and examination times, and improve ultrasound image quality.
- In conventional US imaging, the operator manually moves the transducer, obtaining a series of two-dimensional (2D) images of the target region. Then, he/she performs a mental 3D reconstruction of the underlying anatomy based on the content and the movement patterns used. Physicians have defined a set of protocols to obtain standard views that contain most of the relevant features in the 2D cutting plane of the organ. However, such views are not easy to locate for a person with limited training.
-
Ultrasound images are noisy and are affected by signal dropout, attenuation, speckle, and acoustic shadows. So, the operator does not always perceive the anatomy as clearly as one would like. Moreover, a soft-textured image free of noise due to excessive filtering or incorrect gain settings is useless from a clinical point of view.
-
The application of machine learning techniques to ultrasound image formation has a large margin of improvement to provide less blurry and more significant images, even by creating new ultrasound medical imaging modalities [11]. New approaches in beamforming, super-resolution, and image enhancement require modifications to hardware elements, which are usually more ambitious than more straightforward software post-processing approaches. In spite of the more difficult adoption, many research advances are overcoming traditional and simple reconstruction algorithms, which translate physical measurements of ultrasound waves into images in the display. The more computational power available in medical devices, the more sophisticated real-time solutions may enrich the different ultrasound imaging modalities.
-
AI algorithms can help to reduce the steep learning curve in ultrasound scanning by assisting physicians, nurses, and technicians to perform full examinations, as is shown in detail in the following sections.
-
Image processing algorithms for measurement, quantification, and computer-aided detection have left behind traditional feature engineering, and the most competitive solutions in ultrasound imaging take advantage of deep learning-based approaches.
-
Finally, computer-assisted diagnosis, triage, detection, and quantification have also attracted the research community’s attention because of their significant impact on reducing physicians’ workload.
5. Computer-Assisted Ultrasound Scanning
As indicated above, ultrasound imaging is highly operator-dependent. Therefore, sonographers must be properly trained to be able to acquire clinically useful images. Their training process has a steep learning curve, which is mainly caused by these factors:
-
The operator must place the transducer on the patient’s body surface at precise locations to allow the signal to reach the desired organ and bounce back to the sensor. Bones (such as the ribs) and certain organs (such as the lungs) are opaque to pressure waves, so they must be avoided to reach the target organ.
-
Both the exact location of the acoustic windows and the force to be exerted with the probe slightly depend on the patient’s morphology.
Because of these unique challenges, computer-assisted scanning is a promising set of technologies that may democratize ultrasound medical imaging. Medical practitioners will benefit from any improvements in the effectiveness and autonomy of the acquisition workflow of this imaging modality. The smart guidance for assisted scanning will simplify their work, increase their productivity, decrease their expenses, and optimize their workflow.
Its impact on developing countries can be even bigger. Because there are not enough skilled operators in developing countries, many women do not get any US exams during their pregnancy [12]. Creating a system that can help reduce the level of expertise needed during scanning could have a very significant impact. With this kind of system, any person with a basic anatomical background in a remote area would be able to do an ultrasound exam. Only images with clinical value would be sent to radiologist experts to be evaluated, no matter where the physician lives.
References
- Warner, H.R.; Gardner, R.M.; Toronto, A.F. Computer-Based Monitoring of Cardiovascular Functions in Postoperative Patients. Circulation 1968, 37, II68–II74.
- Ambinder, E.P. A History of the Shift Toward Full Computerization of Medicine. J. Oncol. Pract. 2005, 1, 54–56.
- Turing, A.M. Intelligent Machinery, 1948, 4. Reprinted in Mechanical Intelligence (Collected Works of AM Turing); North-Holland Publishing Co.: Amsterdam, The Netherlands, 1992.
- Keane, P.A.; Topol, E.J. With an Eye to AI and Autonomous Diagnosis. NPJ Digit. Med. 2018, 1, 1–3.
- Do, S.; Song, K.D.; Chung, J.W. Basics of Deep Learning: A Radiologist’s Guide to Understanding Published Radiology Articles on Deep Learning. Korean J. Radiol. 2020, 21, 33–41.
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56.
- Littmann, M.; Selig, K.; Cohen-Lavi, L.; Frank, Y.; Hönigschmid, P.; Kataka, E.; Mösch, A.; Qian, K.; Ron, A.; Schmid, S. Validity of Machine Learning in Biology and Medicine Increased through Collaborations across Fields of Expertise. Nat. Mach. Intell. 2020, 2, 18–24.
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; Van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A Review of Deep Learning in Medical Imaging: Imaging Traits, Technology Trends, Case Studies With Progress Highlights, and Future Promises. Proc. IEEE 2021, 109, 820–838.
- Wang, Y.; Ge, X.; Ma, H.; Qi, S.; Zhang, G.; Yao, Y. Deep Learning in Medical Ultrasound Image Analysis: A Review. IEEE Access 2021, 9, 54310–54324.
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep Learning in Medical Ultrasound Analysis: A Review. Engineering 2019, 5, 261–275.
- Deffieux, T.; Demené, C.; Tanter, M. Functional Ultrasound Imaging: A New Imaging Modality for Neuroscience. Neuroscience 2021, 474, 110–121.
- Shah, S.; Bellows, B.A.; Adedipe, A.A.; Totten, J.E.; Backlund, B.H.; Sajed, D. Perceived Barriers in the Use of Ultrasound in Developing Countries. Crit. Ultrasound J. 2015, 7, 1–5.
More
