Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rahul Shukla and Version 2 by Conner Chen.
Japanese encephalitis virus (JEV) is the causal agent behind Japanese encephalitis (JE), a potentially severe brain infection that spreads through mosquito bites. JE is predominant over the Asia-Pacific Region and has the potential to spread globally with a higher rate of morbidity and mortality. Efforts have been made to identify and select various target molecules essential in JEV’s progression.
- Japanese encephalitis (JE)
- antiviral
- vaccine
- drug
1. Introduction
Japanese encephalitis virus (JEV) is the predominant cause of viral encephalitis in Asia [1]. It is a mosquito-borne flavivirus that is the causative agent of Japanese encephalitis [2]. Japanese encephalitis (JE) was reported for the first time in 1871, in Japan, and JEV was first isolated in 1935 from the brain of a fatal case of JE. This isolate, known as the Nakayama strain, is acknowledged as the prototype strain of JEV [3]. Other clinically relevant viruses belonging to the same genus include dengue virus (DENV), yellow fever virus (YFV) Murray Valley encephalitis (MVE), West Nile virus (WNV), zika virus (ZIKV), St. Louis encephalitis virus (SLEV) and tick-borne encephalitis virus (TBEV) [4]. According to a WHO report published in 2019, almost 68,000 cases of JE with a 20–30% mortality rate were recorded annually. A study based on mathematical modeling with age-stratified case data estimated that approximately 100,308 clinical cases and 20,000–30,000 deaths occurred due to JEV in 2015 across the globe [5]. Newborns and children up to the age of 15 years are more vulnerable to JE with the increased threat of neurological complications over adults [6]. Almost 2 billion people living in endemic countries face a constant threat of JE and an upsurge in the mosquito population poses a risk of expansion to newer geographical areas.
The JEV is an RNA virus, primarily transmitted through the bite of an infected female mosquito, Culex tritaeniorhynchus. Other Culex species, such as Cx. annulirostris, Cx. vishnui, Cx. pseudovishnui, Cx. gelidus, Cx. sitiens and Cx. fuscocephela are also reported to be involved in the transmission of JEV along with some Anopheles mosquito species, such as Anopheles subpictus, An. peditaeniatus and An. hyrcanus [7]. The primary reservoirs of JEV are the birds of the family Ardeidae, such as herons and egrets. Pigs are highly susceptible to JEV, where the virus becomes amplified in optimum levels and they develop a high circulating viral titer (amplified host), and are therefore able to spread the infection to naive mosquitoes [8]. Reports suggest that at this stage, pigs tend to shed the virus in oronasal secretion and may potentiate the horizontal transmission of JEV [9]. Unlike pigs and birds; humans, cattle and horses do not develop high viral titers, making them ‘dead-end’ hosts, as shown in Figure 1.
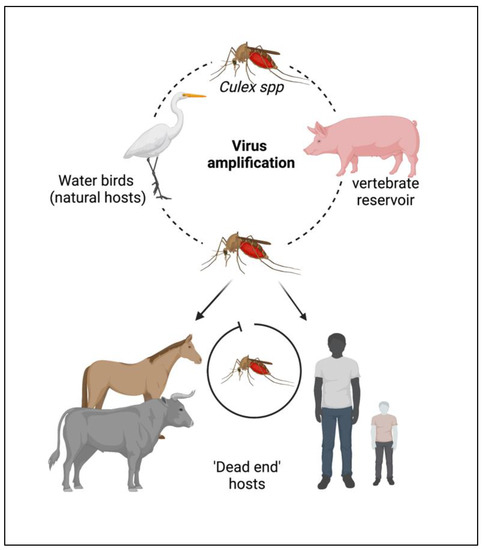
Figure 1. Cycle of the Japanese encephalitis virus (JEV) infection and amplification. Long-legged water birds, such as herons, storks, and ibises are the primary reservoirs and natural hosts for JEV. JEV-infected female mosquitoes, especially Culex tritaeniorhynchus, transmit the virus from wading water birds to other animals (pigs, cattle, and other hooved animals) and humans. Pigs act as secondary hosts where the virus becomes amplified at an optimum level and carries the infectious virion from one place to another (vector-free transmission). Female Culex mosquitos take up the virus from here and infect humans by biting them. The infected humans act as ‘dead-end’ hosts for the virus as JEV does not develop a titer high enough in the blood circulation to transmit through feeding mosquitoes.
Nonetheless, JEV has an enzootic cycle, due to which the virus can persist in nature to such an extent that it might be next to impossible to eradicate it in the near future. Thus, effective antiviral therapy and an ideal vaccine against JEV are of pressing priority. Despite challenges, such as the lack of an appropriate drug delivery system or a treatment plan independent of the stage of infection, JE research has increased rapidly with growing technological advancements, with the primary aim of developing a safe and cost-effective therapeutic (drugs and vaccines) for all age groups.
2. Epidemiology
The majority of cases of viral encephalitis in the Asian subcontinent are due to JEV [6]. This spans a large region that includes majorly tropical parts of Asia, such as Japan, China, Taiwan, Korea, the Philippines, India and all of Southeastern Asia. Countries with confirmed JE epidemics include India, Nepal, Pakistan, Sri Lanka, Myanmar, Laos, Vietnam, Malaysia, the Philippines, Singapore, China, Indonesia, maritime Siberia, Japan and Korea [10]. Additionally, sporadic outbreaks in the Western Pacific and northern Australia are also observed [6]. Historically, outbreaks similar to JE had been recorded in Japan in the late 1800s; however, the first confirmed JE case was documented in 1924 in Japan, followed by Korea (1933), China (1940), the Philippines (1950), India (1955) and many other Asian countries thereafter [5]. In recent decades, geographical hotspots for JE incidences have shifted considerably from South Asian countries (e.g., Japan, South Korea and Taiwan) to South East Asian countries, including Bangladesh, Cambodia, India, Indonesia and Pakistan (Figure 2).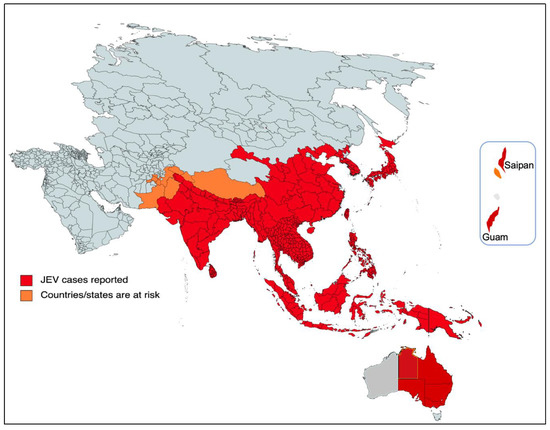
Figure 2. Geographical distribution of JEV. The red part of the focused Indo-Pacific geographical regions and countries indicates where active JEV cases have been reported since its outbreak. The orange color shows the areas that have the highest risk of JEV infection in the near future. The epidemiological data of JEV was modified from https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 26 December 2022).
