Macroion assemblies form an efficient scaffold for GF adsorption. Such assemblies enable the targeted delivery of these proteins without losing their activity. Specific attention is given to three types of growth factors: vascular endothelial growth factors, human fibroblast growth factors, and neurotrophins, as well as selected biocompatible synthetic macroions (obtained through standard polymerization techniques) and polysaccharides (natural macroions composed of repeating monomeric units of monosaccharides). Understanding the mechanisms by which growth factors bind to potential carriers could lead to more effective delivery methods for these proteins, which are of significant interest in the diagnosis and treatment of neurodegenerative and civilization diseases, as well as in the healing of chronic wounds.
- polyelectrolytes
- polysaccharides
- vascular endothelial growth factor
- human fibroblast growth factors
1. PAH-Based Assemblies
2. BPEI-Based Assemblies
3. PAMAM Dendrimer-Based Assemblies
4. PAE and PAA-Based Assemblies
5. CS-Based Assemblies
6. Heparin-Based Assemblies
7. ChS-Based Assemblies
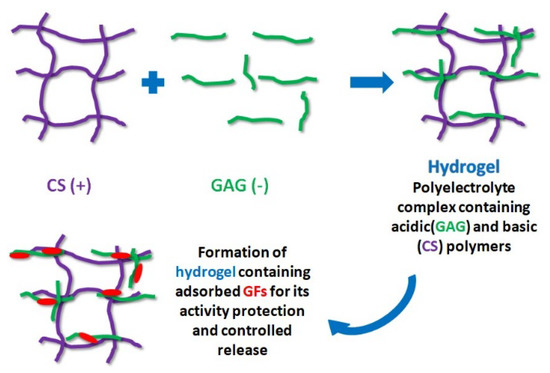
8. Hydrogel-Based Polysaccharides Containing GFs
References
- Müller, S.; Koenig, G.; Charpiot, A.; Debry, C.; Voegel, J.C.; Lavalle, P.; Vautier, D. VEGF-Functionalized Polyelectrolyte Multilayers as Proangiogenic Prosthetic Coatings. Adv. Funct. Mater. 2008, 18, 1767–1775.
- Vodouhê,, C.; Schmittbuhl, M.; Boulmedais, F.; Bagnard, D.; Vautier, D.; Schaaf, P.; Egles, C.; Voegel, J.C.; Ogier, J. Effect of Functionalization of Multilayered Polyelectrolyte Films on Motoneuron Growth. Biomater. 2005, 26, 545–554.
- Zhang, C.-B.; Cao, H.-L.; Qian, L.; Tu, J.; Guo, X.; Liu, Z.; Dong, Z. Enhancement Effect of Ultrasound-Induced Microbubble Cavitation on Branched Polyethylenimine-Mediated VEGF(165) Transfection with Varied N/P Ratio. Ultrasound Med. Biol. 2013, 39, 161–171.
- Won, Y.W.; McGinn, A.N.; Lee, M.; Nam, K.; Bull, D.A.; Kim, S.W. Post-Translational Regulation of a Hypoxia-Responsive VEGF Plasmid for the Treatment of Myocardial Ischemia. Biomaterials 2013, 34, 6229–6238.
- Huang, M.; Vitharana, S.N.; Peek, L.J.; Coop, T.; Berkland, C. Polyelectrolyte Complexes Stabilize and Controllably Release Vascular Endothelial Growth Factor. Biomacromolecules 2007, 8, 1607–1614.
- de Paz, J.L.; Noti, C.; Böhm, F.; Werner, S.; Seeberger, P.H. Potentiation of Fibroblast Growth Factor Activity by Synthetic Heparin Oligosaccharide Glycodendrimers. Chem. Biol. 2007, 14, 879–887.
- Thomas, T.P.; Shukla, R.; Kotlyar, A.; Kukowska-Latallo, J.; Baker, J.R., Jr. Dendrimer-Based Tumor Cell Targeting of Fibroblast Growth Factor-1. Bioorg. Med. Chem. Lett. 2010, 20, 700–703.
- Kwon, M.J.; An, S.; Choi, S.; Jung, H.S.; Chang, S.; Ko, J.H.; Hye, J.; Kim, T.K.; Soo, J.; Park, J.H.; et al. Effective Healing of Diabetic Skin Wounds by Using Nonviral Gene Therapy Based on Minicircle Vascular Endothelial Growth Factor DNA and a Cationic Dendrimer. J. Gene Med. 2012, 14, 272–278.
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel Design of (PEG-ylated ) PAMAM - Based Nanoparticles for Sustained Delivery of BDNF to Neurotoxin-Injured Differentiated Neuroblastoma Cells. J. Nanobiotechnol. 2020, 18, 120.
- Dąbkowska, M.; Rogińska, D.; Kłos, P.; Sobuś, A.; Adamczak, M.; Litwińska, Z.; Machalińska, A.; Machaliński, B. Electrostatic Complex of Neurotrophin 4 with Dendrimer Nanoparticles: Controlled Release of Protein in Vitro and in Vivo. Int. J. Nanomed. 2019, 14, 6117–6131.
- Lee, J.; Jung, J.; Kim, Y.J.; Lee, E.; Choi, J.S. Gene Delivery of PAMAM Dendrimer Conjugated with the Nuclear Localization Signal Peptide Originated from Fibroblast Growth Factor 3. Int. J. Pharm. 2014, 459, 10–18.
- Emamian, M.; Abbaspour, A.; Shahani, T.; Biglari, A.; Sharafi, A. Non-Viral Suicide Gene Therapy: Cytosine Deaminase Gene Directed by VEGF Promoter and 5-Fluorocytosine as a Gene Directed Enzyme/Prodrug System in Breast Cancer Model. Drug Res. 2021, 71, 395–406.
- Backer, M.V.; Gaynutdinov, T.I.; Patel, V.; Bandyopadhyaya, A.K.; Thirumamagal, B.T.S.; Tjarks, W.; Barth, R.F.; Claffey, K.; Backer, J.M. Vascular Endothelial Growth Factor Selectively Targets Boronated Dendrimers to Tumor Vasculature. Mol. Cancer Ther. 2005, 4, 1423–1429.
- Han, U.; Park, H.H.; Kim, Y.J.; Park, T.H.; Park, J.H.; Hong, J. Efficient Encapsulation and Sustained Release of Basic Fibroblast Growth Factor in Nanofilm: Extension of the Feeding Cycle of Human Induced Pluripotent Stem Cell Culture. ACS Appl. Mater. Interfaces 2017, 9, 25087–25097.
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable Dual Growth Factor Delivery from Polyelectrolyte Multilayer Films. Biomaterials 2011, 32, 6183–6193.
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667.
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084.
- Ho, Y.C.; Mi, F.L.; Sung, H.W.; Kuo, P.L. Heparin-Functionalized Chitosan–Alginate Scaffolds for Controlled Release of Growth Factor. Int. J. Pharm. 2009, 376, 69–75.
- Shi, S.; Cheng, X.; Wang, J.; Zhang, W.; Peng, L.; Zhang, Y. RhBMP-2 Microspheres-Loaded Chitosan/Collagen Scaffold Enhanced Osseointegration: An Experiment in Dog. J. Biomater. Appl. 2009, 23, 331–346.
- De la Riva, B.; Sánchez, E.; Hernández, A.; Reyes, R.; Tamimi, F.; López-Cabarcos, E.; Delgado, A.; vora, C. Local Controlled Release of VEGF and PDGF from a Combined Brushite–Chitosan System Enhances Bone Regeneration. J. Control. Release 2010, 143, 45–52.
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672.
- MacDonald, M.L.; Rodriguez, N.M.; Shah, N.J.; Hammond, P.T. Characterization of Tunable FGF-2 Releasing Polyelectrolyte Multilayers. Biomacromolecules 2010, 11, 2053–2059.
- Mao, Z.; Ma, L.; Zhou, J.; Gao, C.; Shen, J. Bioactive Thin Film of Acidic Fibroblast Growth Factor Fabricated by Layer-by-Layer Assembly. Bioconjug. Chem. 2005, 16, 1316–1322.
- Kumorek, M.; Kubies, D.; Riedel, T. Protein Interactions with Quaternized Chitosan/Heparin Multilayers. Physiol. Res. 2016, 65, S253–S261.
- Kumorek, M.; Kubies, D.; Filová, E.; Houska, M.; Kasoju, N.; Chánová, E.M.; Matějka, R.; Krýslová, M.; Bačáková, L.; Rypáček, F. Cellular Responses Modulated by FGF-2 Adsorbed on Albumin/Heparin Layer-by-Layer Assemblies. PLoS ONE 2015, 10, e0125484.
- Almodóvar, J.; Bacon, S.; Gogolski, J.; Kisiday, J.D.; Kipper, M.J. Polysaccharide-Based Polyelectrolyte Multilayer Surface Coatings Can Enhance Mesenchymal Stem Cell Response to Adsorbed Growth Factors. Biomacromolecules 2010, 11, 2629–2639.
- De Cock, L.J.; De Koker, S.; De Vos, F.; Vervaet, C.; Remon, J.P.; De Geest, B.G. Layer-by-Layer Incorporation of Growth Factors in Decellularized Aortic Heart Valve Leaflets. Biomacromolecules 2010, 11, 1002–1008.
- Sandoval, A.M.; Frederik, C.; John, C. Biomimetic Surface Delivery of NGF and BDNF to Enhance Neurite Outgrowth. Biotechnol. Bioeng. 2020, 117, 3124–3135.
- Oliveira, S.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Layer-by-Layer Assembled Cell Instructive Nanocoatings Containing Platelet Lysate. Biomaterials 2015, 48, 56–65.
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Controlled Release of Nerve Growth Factor from a Heparin-Containing Fibrin-Based Cell Ingrowth Matrix. J. Control. Release 2000, 69, 149–158.
- Tezcaner, A.; Hicks, D.; Boulmedais, F.; Sahel, J.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Polyelectrolyte Multilayer Films as Substrates for Photoreceptor Cells. Biomacromolecules 2006, 7, 86–94.
- Ma, L.; Zhou, J.; Gao, C.; Shen, J. Incorporation of Basic Fibroblast Growth Factor by a Layer-by-Layer Assembly Technique to Produce Bioactive Substrates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 285–292.
- Sun, C.; Liu, M.; Sun, P.; Yang, M.; Yates, E.A.; Guo, Z.; Fernig, D.G. Sulfated Polysaccharides Interact with Fibroblast Growth Factors and Protect from Denaturation. FEBS Open Bio 2019, 9, 1477–1487.
- Liu, H.; Zhao, Y.; Zou, Y.; Huang, W.; Zhu, L.; Liu, F.; Wang, D.; Guo, K.; Hu, J.; Chen, J.; et al. Heparin-Poloxamer Hydrogel-Encapsulated RhFGF21 Enhances Wound Healing in Diabetic Mice. FASEB J. 2019, 33, 9858–9870.
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled Release of Fibroblast Growth Factors and Heparin from Photocrosslinked Chitosan Hydrogels and Subsequent Effect on in Vivo Vascularization. J. Biomed. Mater. Res. A 2003, 64, 551–559.
- Obara, K.; Ishihara, M.; Ishizuka, T.; Fujita, M. Photocrosslinkable Chitosan Hydrogel Containing Fibroblast Growth Factor-2 Stimulates Wound Healing in Healing-Impaired Db / Db Mice. Biomaterials 2003, 24, 3437–3444.
- Freudenberg, U.; Zieris, A.; Chwalek, K.; Tsurkan, M.V.; Maitz, M.F.; Atallah, P.; Levental, K.R.; Eming, S.A.; Werner, C. Heparin Desulfation Modulates VEGF Release and Angiogenesis in Diabetic Wounds. J. Control. Release 2015, 220, 79–88.
- Cai, S.; Liu, Y.; Shu, X.Z.; Prestwich, G.D. Injectable Glycosaminoglycan Hydrogels for Controlled Release of Human Basic Fibroblast Growth Factor. Biomaterials 2005, 26, 6054–6067.
- Ding, I.; Peterson, A.M. Half-Life Modeling of Basic Fibroblast Growth Factor Released from Growth Factor-Eluting Polyelectrolyte Multilayers. Sci. Rep. 2021, 11, 9808.
- Jha, A.K.; Mathur, A.; Svedlund, F.L.; Ye, J.; Yeghiazarians, Y.; Healy, K.E. Molecular Weight and Concentration of Heparin in Hyaluronic Acid-Based Matrices Modulates Growth Factor Retention Kinetics and Stem Cell Fate. J. Control. Release 2015, 209, 308–316.
- Parajó, Y.; D’Angelo, I.; Welle, A.; Garcia-Fuentes, M.; Alonso, M.J. Hyaluronic Acid/Chitosan Nanoparticles as Delivery Vehicles for VEGF and PDGF-BB. Drug Deliv. 2010, 17, 596–604.
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; Cryan, S.A. Translational Studies on the Potential of a Vegf Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779.
- Liu, L.S.; Ng, C.K.; Thompson, A.Y.; Poser, J.W.; Spiro, R.C. Hyaluronate-Heparin Conjugate Gels for the Delivery of Basic Fibroblast Growth Factor (FGF-2). J. Biomed. Mater. Res. 2002, 62, 128–135.
- Ehsanipour, A.; Sathialingam, M.; Rad, L.M.; de Rutte, J.; Bierman, R.D.; Liang, J.; Xiao, W.; Carlo, D.D.; Seidlits, S.K. Injectable, Macroporous Scaffolds for Delivery of Therapeutic Genes to the Injured Spinal Cord Injectable, Macroporous Scaffolds for Delivery of Therapeutic Genes to the Injured Spinal Cord. APL Bioeng. 2021, 5, 016104.
