You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
Glutathione is a naturally occurring compound that plays a crucial role in the cellular response to oxidative stress through its ability to quench free radicals, thus mitigating the risk of potential damage, including cell death. While glutathione is endogenously present in different plants and animal cells, their concentration varies considerably. The alteration in glutathione homeostasis can be used as a potential marker for human diseases. In the case of the depletion of endogenous glutathione, exogenous sources can be used to replenish the pool. To this end, both natural and synthetic glutathione can be used.
- glutathione
- oxidative stress
- food
1. Role of Glutathione in Food
Glutathione plays a pivotal role in physiological processes, including in maintaining redox balance, reducing oxidative stress, removing toxins, and regulating immune system functions. The state and concentration of glutathione in the body is considered a vital sign and a therapeutic target for many chronic and age-related diseases. It has been postulated that glutathione levels can be improved in humans by using fruits and vegetables containing glutathione or amino acids that help its (glutathione) synthesis [1]. Eating green foods, including asparagus, avocado, cucumber, green beans, and spinach raw or slightly steamed, is preferable to preserve both forms of glutathione (GSH and GSSG). Studies have shown that dairy products and cereals are low in glutathione, while fruits and vegetables contain moderate to relatively high amounts of glutathione [1][2]. However, processing, preservation, and cooking methods can alter the glutathione content in food products [2]. In addition to health benefits, glutathione is also responsible for the odor and taste of processed food (e.g., GSH induce a sulfurous odor in processed food) [3][4].
The role of glutathione and its two forms in processed food continues to gain considerable attention, primarily in the development of a better understanding and improve the quality of developed products [5][6]. For example, both GSH and GSSG have been shown to improve the gas-retaining properties of rice batter used in gluten-free bread manufacturing along with the retention of viscoelastic properties (in the obtained dough). Sensory tests revealed that GSSG bread has a significantly less sulfurous odor compared to GSH bread (which has been attributed to the presence of hydrogen sulfide and methyl mercaptan in the GSH bread headspace) [4][7]. The similar effect of GSH on elasticity was also reported on wheat dough. Verheyen et al. [8] used GSH as a replacement to rising yeast (Saccharomyces cerevisiae) in wheat dough. They observed a significant softening of the dough after 3 h of fermentation when using a yeast-equivalent amount of GSH instead of active yeast, thus leading the way of potentially replacing dry yeast in bread making. The impact of glutathione in the fermentation food industry is widespread. For example, glutathione supplementation has been shown to impact the stress protection and growth promotion of several lactic acid bacteria species (widely used in the modern fermented food industry and probiotic-based therapeutics) [5]. Supplemented glutathione prevents against all kinds of stress factors, including oxidative, acid, cold, and osmotic stress [5]. Furthermore, the role of glutathione in wine maturation and quality (in terms of a change in odor and taste during storage) have been well documented, regardless of the grape and wine variety [9][10][11]. Taken together, these studies highlight that the supplementation of GSH can bring a dramatic change in the final properties of the processed food and thus play a crucial role in the global food industry. Based on these reports, food can be used as a delivery medium to dose humans with glutathione by using processed food (fortified or supplemented with glutathione). This is particularly useful considering the crucial role glutathione plays in human health and a wide range of diseases.
2. Role of Glutathione in Human Diseases
Glutathione plays a pivotal role in physiological processes, including the maintenance of redox balance, neutralizing oxidative stress by promoting the metabolic detoxification of both xenobiotic and endogenous compounds, and regulating the function of the immune system [12]. The depletion of glutathione leads to the (i) release of inflammatory cytokines, (ii) the formation of free radicals, and (iii) the inhibition of some cell functions, all of which have been known to cause many chronic degenerative diseases and functional loss with ageing. Further, glutathione metabolism dysregulation has been shown to induce diseases to the central nervous system, frailty and sarcopenia, infections, chronic liver diseases, metabolic diseases, pulmonary, and cardiovascular diseases [12]. Glutathione contributes to the detoxification of living organisms (by neutralizing oxygen species (ROS)), regulates cell proliferation, and is involved in immune function. Knowing the concentration of glutathione makes it possible to detect early diseases because of its significant role in regulating cellular stress [13]. Recent studies have shown that glutathione not only affects normal immune function but also participates in complex immune reactions such as fever. The complex role of glutathione was discovered in patients who do not develop a fever during infection [14]. Generally, fever is associated with oxidative stress; therefore, it was believed that the antioxidant properties of glutathione can reduce its incidence. Studies have shown that even a low glutathione level is sufficient to reduce fever. However, the main problem arises when the primary symptoms of infection (i.e., fever) is not manifested. Therefore, it can be proposed that regardless of fever, patients with infection should be treated with glutathione. The impact of glutathione on fever has been comprehensively reviewed in a recent review by Wrotek et al. [14].
The spread of chronic diseases and premature aging at present has led to increased studies in the field of antioxidants, especially glutathione, because of its importance in reducing these diseases. Nutrients, including antioxidants and glutathione, have been recommended to be taken for a very long time or for a lifetime to have an apparent effect on humans. The high cost of these nutrients remains an inhibitory factor limiting their uptake. However, the uptake of exogeneous glutathione consumed as a supplement is still debated [1]. In the meantime, attempts are being made to increase the amount of glutathione in food, such as animal tissues (meat) through the application of modern genetic and reproductive techniques, and improve the levels of these antigens in animals’ bodies. Thus, it is possible to improve the quality of meat products, extend their storage life, and obtain high-quality meat which contains good proportions of glutathione and other antioxidants important to human consumers [15]. Becker et al. [16] conducted a pilot clinical study on the effect of thiol-containing antioxidants (glutathione, α-lipoic acid, and N-acetylcysteine) on the recovery and survival of malnutrition syndrome kwashiorkor children. Kwashiorkor disease is a severe form of malnutrition reported to be associated with oxidative stress. In their study [16], children suffering from the disease were randomly assigned to either a standard treatment (recommended by the WHO) or one of the three study groups receiving either 2 × 600 mg of reduced glutathione (GSH) or 2 × 50 mg of α-lipoic acid or 2 × 100 mg of N-acetylcysteine per day. In a 20-day follow-up, they observed that GSH and α-lipoic acid supplementation had a strong correlation with patient survival rate, as determined from initial skin lesions, blood glutathione levels, glutathione, and total protein concentrations. This research outlined the therapeutic potential of glutathione supplementation in reducing the incidence of severe acute malnutrition caused by oxidative stress [16]. Manley [17] stated that the diet of people plays a vital role in determining the levels of glutathione inside the body, as it was noted that people whose diet depends on red and white meat have higher levels of glutathione, up to 2.3 μmol/kg compared to 1.9 μmol/kg in vegetarian people. The reason behind this disparity is the low amount of vitamin B12 in meat. When consumed, vitamin B12 from meat regulates the sulfur biochemical pathway to produce glutathione.
Glutathione also plays a critical role in infections of the pulmonary system. Studies have shown that glutathione depletion increases a person’s susceptibility towards infections such as tuberculosis. The depletion of glutathione in peripheral blood mononuclear cells and red blood cells has been observed in tuberculosis patients compared to a healthy control [18]. To mitigate this shortage, patients were supplemented with liposomal glutathione. The supplemented liposomal glutathione significantly enhanced the T-cell response in HIV-positive patients with tuberculosis infection, while simultaneously reducing the level of free radicals and immunosuppressive cytokines (interleukin-10 (IL-10) and the transforming growth factor-β (TGF-β)) relative to the placebo-controlled group [19]. The observed infection control has been reasoned to the anti-mycobacterial effects of glutathione [13]. Glutathione also regulates natural killer (NK) cell activity in innate intracellular bacterial infections in tuberculosis-infected patients. In tuberculosis, the cytolytic activity of NK cells is critically impaired in patients with low glutathione levels. The treatment of such patients with N-acetylcysteine can recover and regain the cytolytic activity of NK cells and their efficiency in tackling tuberculosis infection [13][20][21]. Glutathione also regulates dendritic cell maturation and their function in the differentiation of native T-cells. An increase in glutathione has been shown to upregulate IL-12 production by dendritic cells, where (IL-12) is responsible for T-cells differentiation, with a significant potential in the infection control [13]. Due to the fundamental role of glutathione in multiple immune cell types and their functions, it (glutathione) has been shown to be critical in different infections, including HIV. The role of glutathione in HIV has been reviewed in detail elsewhere [13].
Recently, Polonikov [22] hypothesized the role of glutathione in the severity of COVID-19. Using the clinical data of four patients, they observed an inverse correlation of the amount of glutathione and COVID-19 severity in patients where severe cases had significantly low endogenous glutathione levels, higher ROS, and a higher ROS/glutathione ratio in plasma than patients with mild disease [22]. It was hypothesized that glutathione deficiency can increase the susceptibility for the uncontrolled replication of SARS-CoV-2 (COVID-19) virus, leading to a significant increase in viral loading regardless of other factors such as aging, chronic disease comorbidity, and smoking. In this research, it was proposed that the long-term oral administration or parenteral injection of N-acetylcysteine (a precursor of endogenous glutathione synthesis) could be used as an efficient therapy for COVID-19 patients with a serious illness [22][23][24]. The primary reasons behind the use of N-acetylcysteine over pure glutathione as a therapeutic strategy include the low bioavailability and short half-life (2 min) of glutathione when administered orally or intravenously [25][26]. The reason for its low bioavailability is the rapid degradation of glutathione by intestinal and hepatic gamma-glutamyl transferase [27]. To mitigate intestinal degradation, the orobuccal or sublingual form of glutathione delivery has been developed, which was shown to increase the level of GSH and the GSH/GSSG ratio [27][28].
While most work has implicated glutathione in a restorative and preventative role in the cellular function and pathologies of difference diseases, a fine balance must be maintained in glutathione homeostasis as glutathione has shown to have both protective and pathogenic roles. For example, changes in the glutathione antioxidant system and disruption in its homeostasis have been implicated in tumor initiation, progression, and treatment response [29]. At high concentrations in tumor cells, glutathione has been shown to cause tumor progression and an increased resistance to chemotherapeutic drugs. Therefore, focus has been drawn towards targeting the glutathione antioxidant system in tumor cells as a therapeutic approach using drugs to target glutathione directly, indirectly, or by using glutathione-based prodrugs (Figure 1) [29]. The concept of a therapeutic approach towards glutathione homeostasis in cancer has been recently reviewed by Kennedy et al. [29], and readers interested in this topic are directed to that comprehensive review.
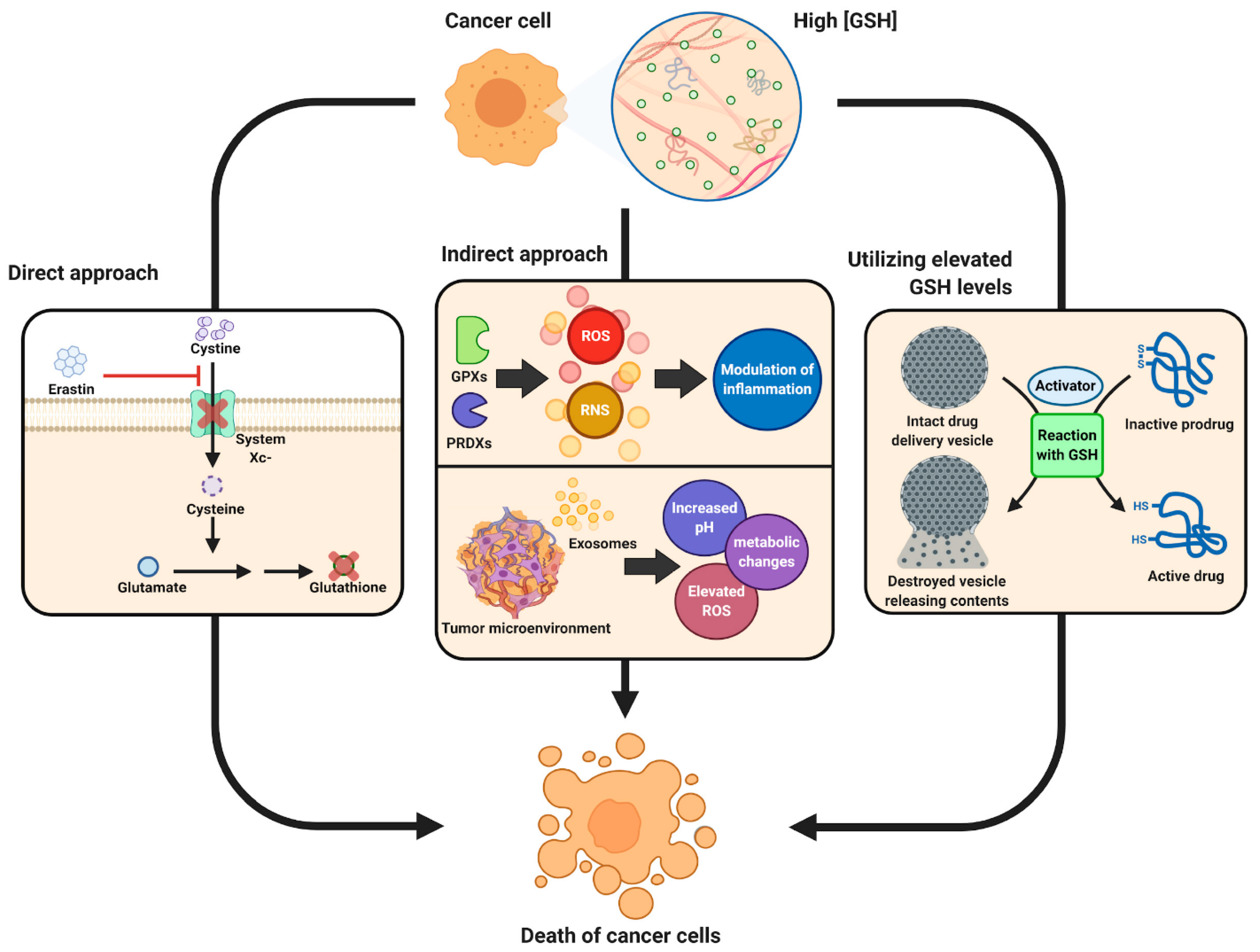
Figure 1. Schematic showing therapeutic role of glutathione in cancer tumor biology. Reproduced with permission from [29].
Glutathione prodrugs or amino acid precursors can also regulate ageing. In ageing, mitochondria start to dysfunction due to the decline in de novo glutathione synthesis, resulting in enhanced oxidative stress, thereby making cells susceptible to microbial infection and death. This compromised mitochondrial function is driven by the combination of a shortfall in glutathione precursor amino acids (cysteine and glycine) and the accumulation of homocysteine (a toxic transsulfuration/glutathione biosynthesis pathway intermediate) [13]. Therefore, it is believed that a supplementation with glutathione precursors can alleviate mitochondrial dysfunction and prevent cell death, restricting ageing. However, the mode of supplementation must be carefully consideredas.
References
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073.
- Jones, D.P.; Coates, R.J.; Flagg, E.W.; Eley, J.W.; Block, G.; Greenberg, R.S.; Gunter, E.W.; Jackson, B. Glutathione in foods listed in the national cancer institute’s health habits and history food frequency questionnaire. Nutr. Cancer 1992, 17, 57–75.
- MECCHI, E.P.; PIPPEN, E.L.; LINEWEAVER, H. Origin of Hydrogen Sulfide in Heated Chicken Muscle. J. Food Sci. 1964, 29, 393–399.
- Yano, H. Comparison of Oxidized and Reduced Glutathione in the Bread-Making Qualities of Rice Batter. J. Food Sci. 2012, 77, C182–C188.
- Pophaly, S.D.; Singh, R.; Pophaly, S.D.; Kaushik, J.K.; Tomar, S.K. Current status and emerging role of glutathione in food grade lactic acid bacteria. Microb. Cell Factories 2012, 11, 114.
- Jänsch, A.; Korakli, M.; Vogel, R.F.; Gänzle, M.G. Glutathione Reductase from Lactobacillus sanfranciscensis DSM20451-T: Contribution to Oxygen Tolerance and Thiol Exchange Reactions in Wheat Sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4469–4476.
- Yano, H. Improvements in the Bread-Making Quality of Gluten-Free Rice Batter by Glutathione. J. Agric. Food Chem. 2010, 58, 7949–7954.
- Verheyen, C.; Albrecht, A.; Herrmann, J.; Strobl, M.; Jekle, M.; Becker, T. The contribution of glutathione to the destabilizing effect of yeast on wheat dough. Food Chem. 2015, 173, 243–249.
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Marcon, Â.R.; Carnieli, G.J.; Vanderlinde, R. Effect of glutathione addition in sparkling wine. Food Chem. 2014, 159, 391–398.
- Vaimakis, V.; Roussis, I.G. Must oxygenation together with glutathione addition in the oxidation of white wine. Food Chem. 1996, 57, 419–422.
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277.
- Novelli, A.; Bianchetti, A. Glutathione: Pharmacological aspects and implications for clinical use. Geriatr. Care 2022, 8.
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Chapter Five—Glutathione as a Marker for Human Disease. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 87, pp. 141–159.
- Wrotek, S.; Sobocińska, J.; Kozłowski, H.M.; Pawlikowska, M.; Jędrzejewski, T.; Dzialuk, A. New Insights into the Role of Glutathione in the Mechanism of Fever. Int. J. Mol. Sci. 2020, 21, 1393.
- Liu, S.M.; Eady, S.J. Glutathione: Its implications for animal health, meat quality, and health benefits of consumers %J Australian Journal of Agricultural Research. Aust. J. Agric. Res. 2005, 56, 775–780.
- Becker, K.; Pons-Kühnemann, J.; Fechner, A.; Funk, M.; Gromer, S.; Gross, H.J.; Grünert, A.; Schirmer, R.H. Effects of antioxidants on glutathione levels and clinical recovery from the malnutrition syndrome kwashiorkor—A pilot study. Redox Rep. 2005, 10, 215–226.
- Manley, R.C. Comparing Glutathione in the Plasma of Vegetarian and Omnivore Populations. Academic Thesis, Arizona State University, Tempe, AZ, USA, 2019.
- Venketaraman, V.; Millman, A.; Salman, M.; Swaminathan, S.; Goetz, M.; Lardizabal, A.; David, H.; Connell, N.D. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 2008, 44, 255–261.
- Judy, L.; Minette, L.; Tommy, S.; Manpreet, K.S.; Enrique, V.T.; Devin, M.; Jessica, A.; John, D.; Cesar, O.; Nishita, P.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887.
- Venketaraman, V.; Dayaram, Y.K.; Talaue, M.T.; Connell, N.D. Glutathione and Nitrosoglutathione in Macrophage Defense against Mycobacterium tuberculosis. Infect. Immun. 2005, 73, 1886–1889.
- Ariel, C.; Millman, M.S.; Yaswant, K.; Dayaram Nancy, D. Connell, and Vishwanath Venketaraman. Natural Killer Cells, Glutathione, Cytokines, and Innate Immunity Against Mycobacterium tuberculosis. J. Interferon Cytokine Res. 2008, 28, 153–165.
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562.
- Poe, F.L.; Corn, J. N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2. Med. Hypotheses 2020, 143, 109862.
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020, 34, 13185–13193.
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220.
- Lomaestro, B.M.; Malone, M. Glutathione in Health and Disease: Pharmacotherapeutic Issues. Ann. Pharmacother. 1995, 29, 1263–1273.
- Buonocore, D.; Grosini, M.; Giardina, S.; Michelotti, A.; Carrabetta, M.; Seneci, A.; Verri, M.; Dossena, M.; Marzatico, F. Bioavailability Study of an Innovative Orobuccal Formulation of Glutathione. Oxidative Med. Cell. Longev. 2016, 2016, 3286365.
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205.
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429.
More
