Cancer is a result of abnormal cell proliferation. This pathology is a serious health problem since it is a leading cause of death worldwide. Anti-cancer therapies rely on surgery, radiation, and chemotherapy. However, these treatments still present major associated problems, namely the absence of specificity. Nanoparticles, particularly dendrimers, have been paving their way to the front line of cancer treatment, mostly for drug and gene delivery, diagnosis, and disease monitoring. This is mainly derived from their high versatility, which results from their ability to undergo distinct surface functionalization, leading to improved performance.
- cancer
- nanocarriers
- anticancer dendrimers
- intracellular targeting
- theranostics
1. Introduction
2. Dendrimer Nanoparticles
Multifunctional nanoparticles display great potential for drug and gene delivery, especially for cancer therapy [11][12]. Dendrimers are a class of hyperbranched synthetic polymers with a very low polydispersity, also known as “cascade polymers” [13]. Biologically, dendrimers are highly biocompatible, with a predictable biodistribution and cell-membrane-interacting features mostly determined by their size and surface charge [14]. They were first synthesized in the late 1970s by Tomalia et al., with the desire to mimic a common pattern in nature with vast potential applications [15]. Due to their hyperbranched structure, dendrimers are extremely versatile macromolecules. Their structure can be defined by three main elements: the inner core, repetitive branching units (dendrons), and terminal groups that provide surface tuning (Figure 1) [16].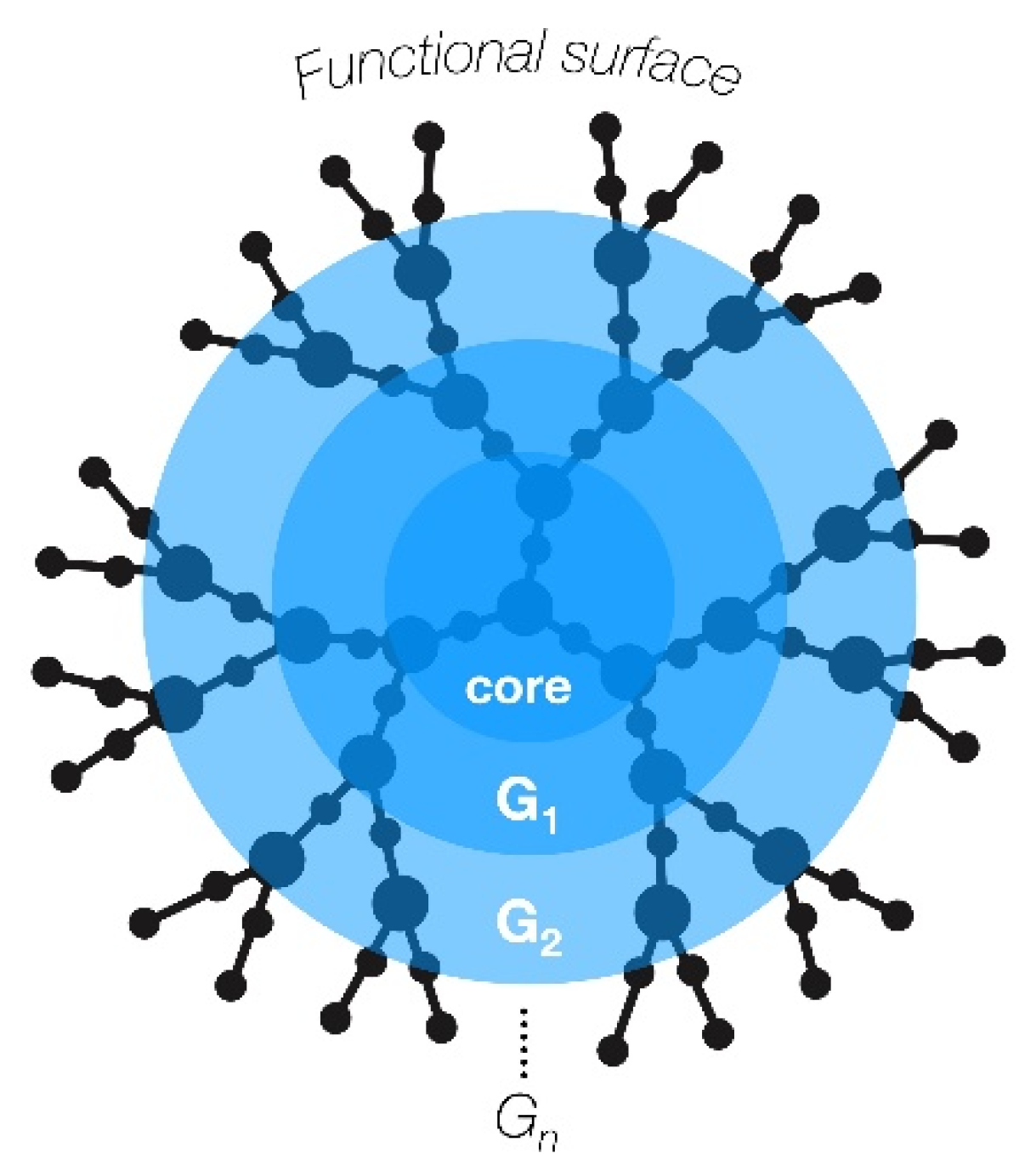
|
Classes of Dendrimers |
Chemical Structure |
|---|---|
|
PAMAM |
Ethylenediamine-based core and terminal groups with primary amines |
|
PPL |
Amino acid lysine-base core and branching units |
|
PPI |
1,4-Diaminobutane-based core and terminal groups with primary amines |
|
Phosphorous dendrimers |
P-Cl-based core, azabisphosphonates are possible terminal groups |
|
Carbosilane dendrimers |
Si-based dendrimer |
3. Dendrimers General Role in Cancer
Dendrimers can be used in a vast number of theranostic applications [26][27][28][29]. It is, therefore, important to be aware of the properties they have to display in order to be employed as biomedical devices. Biocompatibility is crucial to preventing undesirable responses from the host, a property that can only be defined depending on specific applications [29]. In order to prevent bioaccumulation and consequent toxicity, biodegradability is a must. Another important aspect for the development of biomedical devices is their pharmacokinetics, namely their fate in the body after administration [29]. Additionally, the water solubility of dendrimer–drug conjugates enhance the bioavailability of poorly soluble drugs [30]. Lastly, polyvalence, i.e., the ability to support versatile surface functionalization and multiple interactions with biological receptors, is a key property for highly versatile platforms [27]. Dendrimers, as shown in several studies, have a high potential to be used as nanocarriers for both diagnostic and therapeutic approaches [31][32]. Dendrimer–drug interactions might occur in many ways and are dependent on multiple factors such as size, charge, or the chemical nature of the dendrimer/drug. The chemistry behind nanocarriers is the same used in diagnostic or therapeutic schemes, with the agent selected for conjugation being the key player. In general, dendrimers could be used as nanocarriers via two major approaches: loading or conjugation at the surface of the drug and/or target molecule. Encapsulation solves solubility problems indicated by many chemotherapeutics and drugs in general. When a drug is entrapped into the dendrimer’s cavity, the polymer works as a dendritic box [33][34]; in this case, the dendrimers can cargo the drug of interest by forming structures that are stabilized via non-covalent interactions. In a different context, dendrimers could also be used as gene vectors, especially cationic dendrimers. When the strategy is dendrimer–drug conjugation, systemic effects can be reduced, increasing the efficacy of cellular targeting. This strategy also improves the half-life of the drug. The conjugate linker is also key to understanding release mechanisms. In many cases, ester and amide conjugate linkers are used that allow enzymatic or hydrolytic cleavage [34][35], easier for esters than amides [36]. Dendrimer–drug conjugation may influence the efficacy of drug itself. Importantly, these nano-polymers can cross cellular barriers by transcellular or paracellular pathways. To use dendrimers as an alternative diagnostic tool or improve the properties of a contrast agent, it is important to guarantee some criteria. Dendrimers offer many advantages to improve the free delivery of contrast agents or drugs, including high solubility and low polydispersity, which are properties of all dendrimer classes (Table 2).|
Properties |
Observations |
References |
|---|---|---|
|
Low polydispersity index |
Common to all classes of dendrimers |
|
|
EPR effect |
Size/generation/Mw dependent |
[39] |
|
Permeability towards BBB |
Already observed for PAMAM dendrimers |
|
|
Highly solubility |
Common to the majority of dendrimer classes |
|
|
Multifunctional platform |
Common to all classes of dendrimers |
[46] |
|
Highly loading capacity |
Size/generation/Mw dependent |
[47] |
|
Stability |
Common to all classes of dendrimers |
[39] |
|
Low toxicity and immunogenicity |
Size/generation/Mw/charge dependent |
EPR: enhanced permeability and retention, BBB: blood–brain barrier.
4. Dendrimers Cellular Uptake and Mechanism of Action at Cell Organelle Level
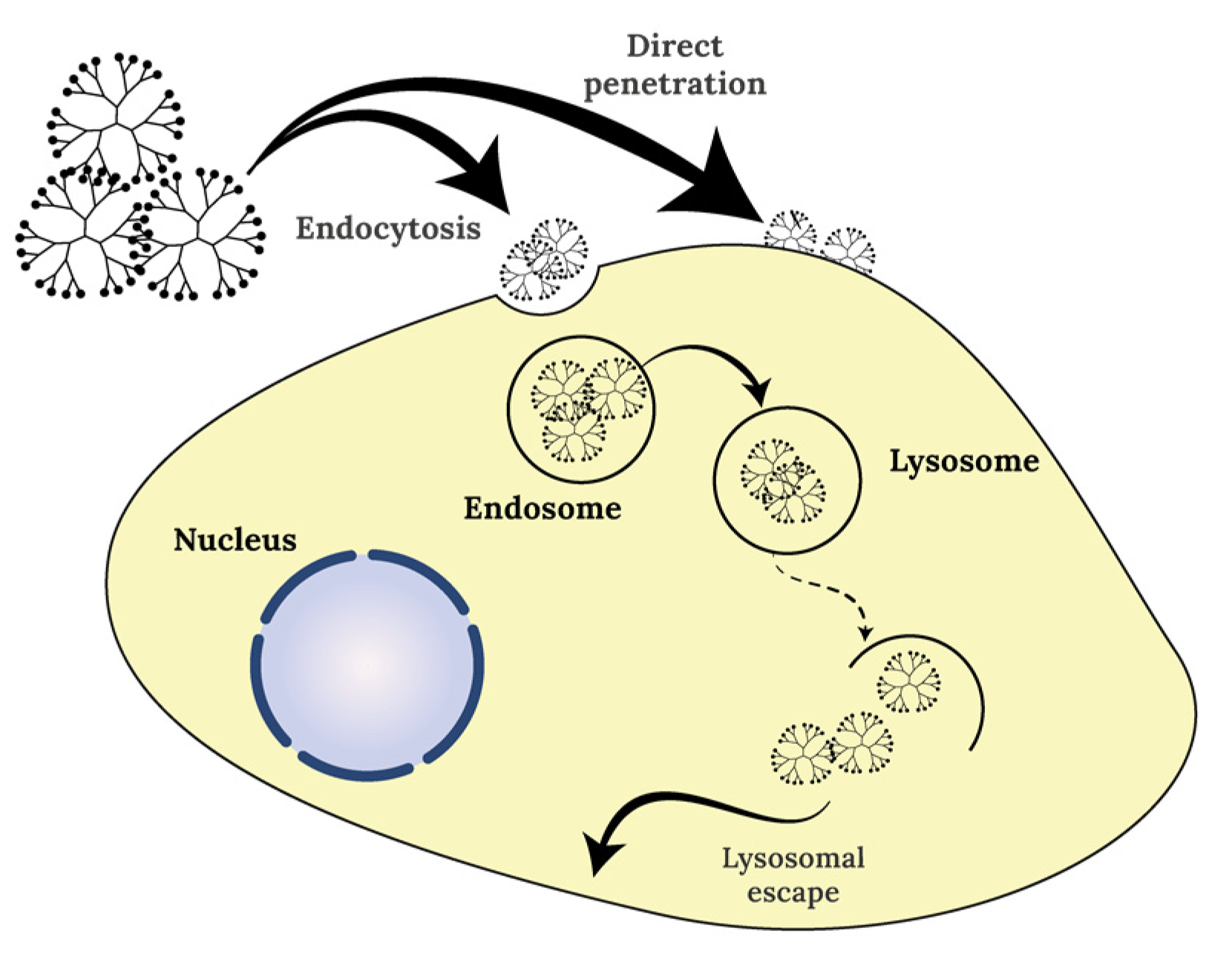
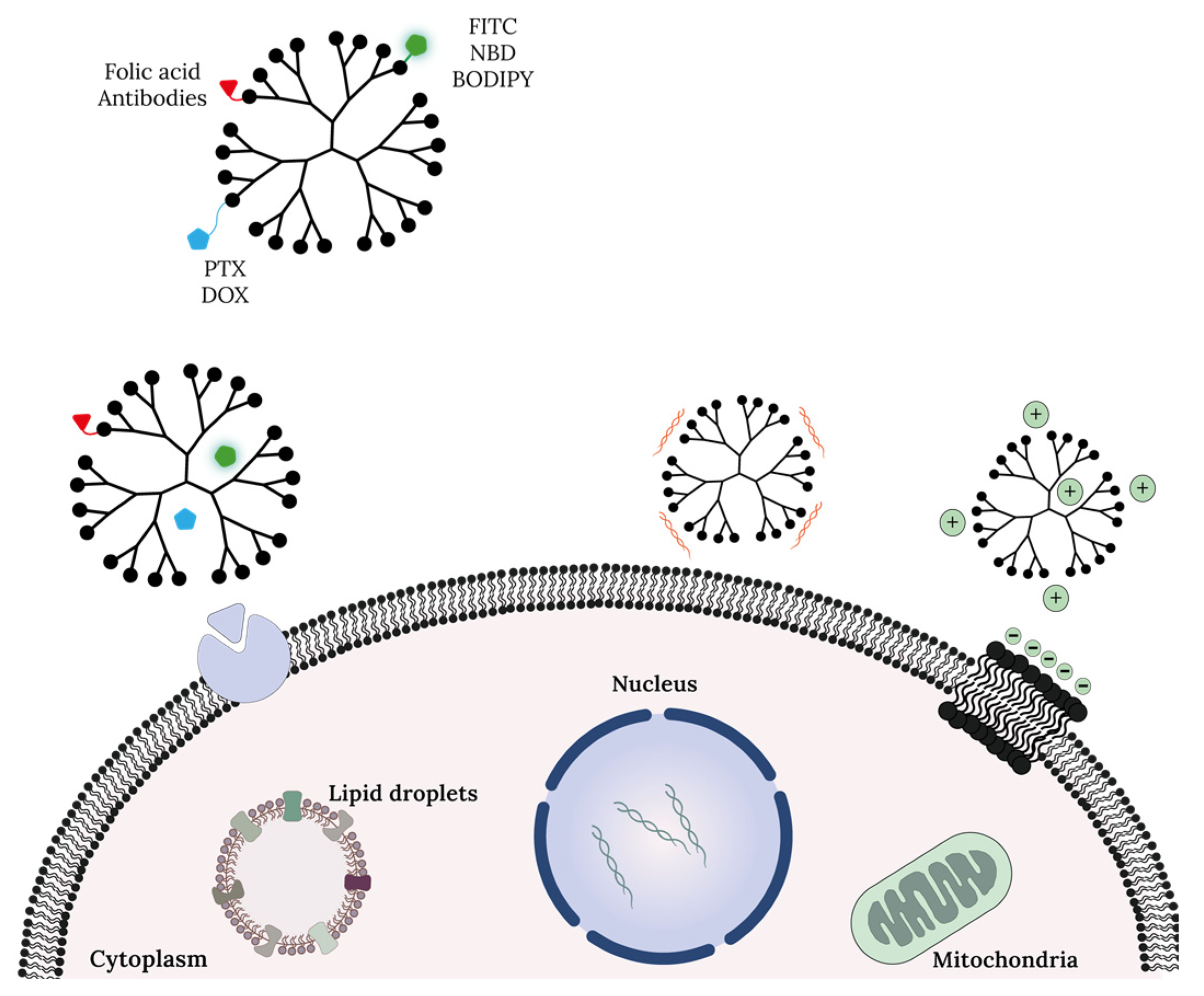
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129.
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419.
- Parsa, N. Environmental factors inducing human cancers. Iran J. Public Health 2012, 41, 1–9.
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm 2015, 93, 52–79.
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A.K.; Thomas, T.; Mulé, J.; Baker, J.R., Jr. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm. Res. 2002, 19, 1310–1316.
- Wiener, E.C.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lauterbur, P.C. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 1994, 31, 1–8.
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75.
- Restani, R.B.; Morgado, P.I.; Ribeiro, M.P.; Correia, I.J.; Aguiar-Ricardo, A.; Bonifácio, V.D. Biocompatible polyurea dendrimers with pH-dependent fluorescence. Angew. Chem. Int. Ed. Engl. 2012, 51, 5162–5165.
- Wu, Y.; Sefah, K.; Liu, H.; Wang, R.; Tan, W. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5–10.
- Shao, S.; Zhou, Q.; Si, J.; Tang, J.; Liu, X.; Wang, M.; Gao, J.; Wang, K.; Xu, R.; Shen, Y. A non-cytotoxic dendrimer with innate and potent anticancer and anti-metastatic activities. Nat. Biomed. Eng. 2017, 1, 745–757.
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433.
- Rodríguez-Acosta, G.L.; Hernández-Montalbán, C.; Vega-Razo, M.F.S.; Castillo-Rodríguez, I.O.; Martínez-García, M. Polymer-dendrimer Hybrids as Carriers of Anticancer Agents. Curr. Drug Targets 2022, 23, 373–392.
- Buhleier, E.; Wehner, W.; VÖGtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158.
- Heegaard, P.M.; Boas, U.; Sorensen, N.S. Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug Chem. 2010, 21, 405–418.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132.
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjug Chem. 2015, 26, 1198–1211.
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647.
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021.
- Dockery, L.; Daniel, M.C. Dendronized Systems for the Delivery of Chemotherapeutics. Adv. Cancer Res. 2018, 139, 85–120.
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 2013, 34, 2252–2264.
- Lo, S.T.; Kumar, A.; Hsieh, J.T.; Sun, X. Dendrimer nanoscaffolds for potential theranostics of prostate cancer with a focus on radiochemistry. Mol. Pharm. 2013, 10, 793–812.
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- Rahimi, A.; Amjad-Iranagh, S.; Modarress, H. Molecular dynamics simulation of coarse-grained poly(L-lysine) dendrimers. J. Mol. Model. 2016, 22, 59.
- Caminade, A.M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333.
- Maroto-Díaz, M.; Elie, B.T.; Gómez-Sal, P.; Pérez-Serrano, J.; Gómez, R.; Contel, M.; Javier de la Mata, F. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium(ii) complexes. Dalton Trans. 2016, 45, 7049–7066.
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526.
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247.
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo de Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of Dendrimers in Drug Delivery Agents, Diagnosis, Therapy, and Detection. J. Nanomater. 2014, 2014, 507273.
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237.
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharm. 2017, 8, 261.
- Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212.
- Trembleau, L.; Simpson, M.; Cheyne, R.W.; Escofet, I.; Appleyard, M.V.C.A.L.; Murray, K.; Sharp, S.; Thompson, A.M.; Smith, T.A.D. Development of 18F-fluorinatable dendrons and their application to cancer cell targeting. New J. Chem. 2011, 35, 2496–2502.
- Liu, M.; Fréchet, J.M. Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 1999, 2, 393–401.
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185.
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18.
- Najlah, M.; Freeman, S.; Attwood, D.; D’Emanuele, A. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int. J. Pharm. 2007, 336, 183–190.
- Kolhe, P.; Khandare, J.; Pillai, O.; Kannan, S.; Lieh-Lai, M.; Kannan, R. Hyperbranched polymer-drug conjugates with high drug payload for enhanced cellular delivery. Pharm. Res. 2004, 21, 2185–2195.
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Chapter 8—Dendrimers as novel drug-delivery system and its applications. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 333–392.
- Rai, D.B.; Gupta, N.; Pooja, D.; Kulhari, H. Dendrimers for diagnostic applications. In Pharmaceutical Applications of Dendrimers; Chauhan, A., Kulhari, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 291–324.
- Zhao, J.; Zhang, B.; Shen, S.; Chen, J.; Zhang, Q.; Jiang, X.; Pang, Z. CREKA peptide-conjugated dendrimer nanoparticles for glioblastoma multiforme delivery. J. Colloid. Interface Sci. 2015, 450, 396–403.
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model. J. Control. Release 2018, 283, 175–189.
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628.
- Srinageshwar, B.; Dils, A.; Sturgis, J.; Wedster, A.; Kathirvelu, B.; Baiyasi, S.; Swanson, D.; Sharma, A.; Dunbar, G.L.; Rossignol, J. Surface-Modified G4 PAMAM Dendrimers Cross the Blood–Brain Barrier Following Multiple Tail-Vein Injections in C57BL/6J Mice. ACS Chem. Neurosci. 2019, 10, 4145–4150.
- Yiyun, C.; Tongwen, X. Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur. J. Med. Chem. 2005, 40, 1188–1192.
- Hawker, C.J.; Wooley, K.L.; Fréchet, J.M.J. Unimolecular micelles and globular amphiphiles: Dendritic macromolecules as novel recyclable solubilization agents. J. Chem. Soc. Perkin Trans. 1993, 1, 1287–1297.
- Vaidya, A.; Jain, S.; Pathak, K.; Pathak, D. Dendrimers: Nanosized Multifunctional Platform for Drug Delivery. Drug Deliv. Lett. 2018, 8, 3–19.
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634.
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142.
- Pinto, S.N.; Mil-Homens, D.; Pires, R.F.; Alves, M.M.; Serafim, G.; Martinho, N.; Melo, M.; Fialho, A.M.; Bonifácio, V.D.B. Core–shell polycationic polyurea pharmadendrimers: New-generation of sustainable broad-spectrum antibiotics and antifungals. Biomater. Sci. 2022, 10, 5197–5207.
- Pryor, J.B.; Harper, B.J.; Harper, S.L. Comparative toxicological assessment of PAMAM and thiophosphoryl dendrimers using embryonic zebrafish. Int. J. Nanomed. 2014, 9, 1947–1956.
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683.
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690.
- Shinde Patil, V.R.; Campbell, C.J.; Yun, Y.H.; Slack, S.M.; Goetz, D.J. Particle diameter influences adhesion under flow. Biophys. J. 2001, 80, 1733–1743.
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271.
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007, 24, 2138–2145.
- Kitchens, K.M.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharm. 2008, 5, 364–369.
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer internalization and intracellular trafficking in living cells. Mol. Pharm. 2010, 7, 680–688.
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of Poly(amidoamine) Dendrimers with Supported Lipid Bilayers and Cells: Hole Formation and the Relation to Transport. Bioconjugate Chem. 2004, 15, 774–782.
- Jahanafrooz, Z.; Mokhtarzadeh, A. Pore-forming Peptides: A New Treatment Option for Cancer. Curr. Med. Chem. 2022, 29, 4078–4096.
- Chernyshova, D.N.; Tyulin, A.A.; Ostroumova, O.S.; Efimova, S.S. Discovery of the Potentiator of the Pore-Forming Ability of Lantibiotic Nisin: Perspectives for Anticancer Therapy. Membranes 2022, 12, 1166.
- Tajarobi, F.; El-Sayed, M.; Rege, B.D.; Polli, J.E.; Ghandehari, H. Transport of poly amidoamine dendrimers across Madin-Darby canine kidney cells. Int. J. Pharm. 2001, 215, 263–267.
- Szwed, A.; Miłowska, K.; Michlewska, S.; Moreno, S.; Shcharbin, D.; Gomez-Ramirez, R.; de la Mata, F.J.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro. Biomolecules 2020, 10, 427.
- Albertazzi, L.; Storti, B.; Marchetti, L.; Beltram, F. Delivery and subcellular targeting of dendrimer-based fluorescent pH sensors in living cells. J. Am. Chem. Soc. 2010, 132, 18158–18167.
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharm. 2010, 248, 259–268.
- Czarnomysy, R.; Muszyńska, A.; Rok, J.; Rzepka, Z.; Bielawski, K. Mechanism of Anticancer Action of Novel Imidazole Platinum(II) Complex Conjugated with G2 PAMAM-OH Dendrimer in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 5581.
- Zhang, X.; Zhang, Z.; Xu, X.; Li, Y.; Li, Y.; Jian, Y.; Gu, Z. Bioinspired therapeutic dendrimers as efficient peptide drugs based on supramolecular interactions for tumor inhibition. Angew. Chem. Int. Ed. Engl. 2015, 54, 4289–4294.
- Antunes, P.; Cruz, A.; Barbosa, J.; Bonifácio, V.D.B.; Pinto, S.N. Lipid Droplets in Cancer: From Composition and Role to Imaging and Therapeutics. Molecules 2022, 27, 991.
- Sharma, A.; Khatchadourian, A.; Khanna, K.; Sharma, R.; Kakkar, A.; Maysinger, D. Multivalent niacin nanoconjugates for delivery to cytoplasmic lipid droplets. Biomaterials 2011, 32, 1419–1429.
