Antioxidants are a class of a multitude of chemical substances clearly associated with large health benefits and lower risks of various age-related diseases.
2. Biochemistry of Antioxidants and Their Mode of Action
Endogenous antioxidants are body products. In contrast with the exogenous antioxidants, the body possesses enzyme systems with an antioxidant action (superoxide dismutase, glutathione peroxidase, and catalysis), co-participating in the deactivation of some free radicals that are formed in the body
[4][5][30,31]. As a defense against oxidative stress imbalances, the body has produced so-called endogenous antioxidants, enzyme systems capable of annihilating free oxygen molecules, preventing the production of negative effects in the body
[6][7][32,33]. Among the endogenous antioxidants,
we mention superoxide-dismutase, catalase, glutathione peroxidase, and hydropersulfides
are mentioned [8][9][34,35]. Some subtypes of glutathione peroxidase are selenium (Se)-dependent, and recent studies
[10][11][12][13][14][36,37,38,39,40] show that an increased intake of Se is associated with protection against the development of cancer and other chronic diseases.
Antioxidants as food additives are referring to some natural or synthetic (established) antioxidants which are also widely used in the food industry to prevent a reduction in the oxidation of fats or other components present in food, during the preservation period
[15][16][41,42].
Exogenous antioxidants are introduced with food and are referred to the established or natural antioxidants. Because it is much more effective, and cheaper, to maintain good health rather than to regain it, the best protection against free radicals is to build and maintain “antioxidant shields”, through a regimen of adequate food with little fat, rich in digestive fibers, and in antioxidant substances, such as vitamin E, vitamin C, and beta-carotene, combined with regular exercise
[17][18][43,44], and through a life program
[19][45] aimed at avoiding, as much as possible, the situations in which
rwe
searchers are exposed to the attack of free radicals. Foods with a high content of antioxidants constitute the basis of nutritional strategies that
we can take from external sources
can be taken from [20][21][46,47]. Food of plant origin was associated with a high content of antioxidants
[21][22][47,48]. Importantly, exogenous antioxidants that can be taken in the diet have the same role in reducing the excessive number of free radicals. The most important of these external (or exogenous) antioxidants are vitamin C, vitamin E, and beta-carotene
[23][49].
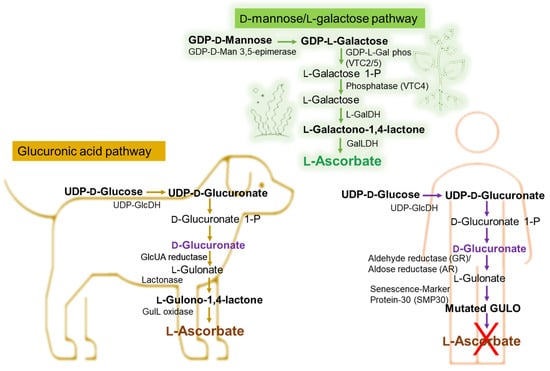
Taking the example of vitamin C’s different pathways to biosynthesis in marine algae or plants, animals, and the human bodies,
we can emphasize the importance of the exogenous addition of antioxidants such as vitamin C in the human body
can be emphasized. The Smirnoff–Wheeler pathway, in which vitamin C is synthesized from D-mannose and L-galactose (D-mannose/L-galactose pathway)
[24][50], represents the major route of vitamin C biosynthesis in marine algae and plants, at the cellular level (
Figure 15), the other three involved routes being the glucose, myoinositol, and the galacturonate pathways
[25][26][27][28][51,52,53,54]. Most animals produce relatively high levels of ascorbic acid from glucose in the liver via the glucuronic acid pathway (
Figure 15)
[29][30][31][32][55,56,57,58]. Humans are unable to synthesize vitamin C and must ingest this vitamin
[33][34][59,60].
Figure 15. Biochemical pathway of vitamin C synthesis in animals vs. plants/green algae and humans. GDP-L-Gal phos, guanosine diphosphate-L-galactose phosphorylase; GalDH, galactose dehydrogenase; UDP-GlcDH, uridine diphosphate glucose dehydrogenase; GlcUA reductase, glucuronic acid reductase; GulL oxidase (GULO), L-gulonolactone oxidase. This figure is based on information from references
[35][36][37][38][39][61,62,63,64,65].
As shown in
Figure 15, in certain vertebrates (i.e., dogs), L-Ascorbate synthesis involves three enzymatic steps starting from the conversion of D-Glucuronate, with L-Gulonate and L-Gulono-γ-lactone (L-Gulono-1,4-lactone) as intermediate metabolites
[35][36][37][38][39][40][61,62,63,64,65,66]. If in this case, the final enzymatic step is catalyzed by L-Gulono-γ-lactone oxidase (GULO), converting L-Gulonate to L-Ascorbate, in humans, the GULO enzyme is mutated (
Figure 15) and not functional in primates also including guinea pigs and some spontaneous mutant mouse and rat models
[39][41][65,67]. Instead, the conversion of L-Gulonate to L-Gulono-1,4-lactone occurs via Senescence-Marker Protein-30 (SMP30) (
Figure 15) also known as regucalcin
[36][39][62,65]. Likewise, in the humans’ case, the conversion of D-Glucuronate to L-Gulonate occurs mainly through aldehyde reductase (GR) and to a smaller extent with aldose reductase’ (AR) contribution (
Figure 15)
[25][39][40][51,65,66]. Considering that the common molecular mechanism of the body’s limited ability to synthesize vitamin C is the absence of GULO
[42][43][44][45][68,69,70,71], genetically, it is considered that the loss of synthesizing the ascorbic acid is likely due to the complete loss of the L-gulono-γ-lactone oxidase (GULO) gene.
Combinations of all these facts bring us to the conclusion of an important balanced diet associated with antioxidant supplements, potentiating each other’s effects and influencing the prevention of diseases, such as heart disease, arthritis, visual impairment, stroke, and premature aging of the skin, enhancing well-being.
Membrane lipids represent a major target of the radical attack, due to the presence of double bonds in the structures of the polyunsaturated fatty acids which comprise them. Membrane phospholipids most frequently contain unsaturated fatty acids, i.e., linoleic acid, linolenic acid, and arachidonic acid
[46][47][27,28]. Membrane lipid peroxidation affects the structure and functions of the plasma membrane and the membranes of intracytoplasmic organelles so that transmembrane potentials, ion fluxes, and transmembrane transport are disturbed, and membrane receptors are inactivated and signaling pathways are deregulated
[45][48][71,72].
The process of lipid peroxidation changes not only the lipid components of membranes but also the proteins, following the reaction of some amino acids with the aldehyde products of peroxidation
[45][71]. Oxidative changes in proteins under the action of reactive oxygen species can also cause the inactivation of enzymes and membrane proteins
[49][73], thereby producing structural changes that lead to the destabilization of cell morphology. The products generated because of lipid peroxidation are involved in inflammatory diseases
[50][74], aging
[51][75], hepatotoxicity
[52][76], hemolysis
[53][77], and all phases of carcinogenesis during the appearance of malignant tumors and metastases
[54][78].
The effect of reactive oxygen species on enzymes includes, for the most part, decreased catalytic capacity, often caused by the oxidation of sulfhydryl groups and the modification of amino groups
[55][56][79,80]. Some free radicals result from normal cellular processes, for example, when cells use oxygen as fuel for energy production, free radicals appear as secondary products of this metabolic process necessary to sustain life
[57][9]. On the other hand, both the environment, in which we live, and the living environment are other main factors causing reactive oxygen species
[57][58][9,12]. Antioxidants can interrupt the sequence of oxidation reactions before it is initiated. In general, antioxidants have a high reduction potential, releasing hydrogen ions, with the inhibition process proceeding as shown in the following representation (Equation (1))
[59][81]:
InH + RO
2−
2
− (1)
where InH is an antioxidant, RO
−2 is a free hydroperoxide radical ion, RO
2H is hydroperoxide of, e.g., a fatty acid, and In
− is an inactive or weakly active radical ion.
But in all cases, with the increase in the inactivation duration, there is a decrease in the number of antioxidants—the increase in the peroxide index is found only after there has been a significant decrease in the added antioxidant
[2][60][61][2,82,83]. Taking here the α-tocopherol (Vitamin E) as an example of lipid-soluble antioxidant, which acts as a “chain breakerf” to intercept lipid peroxyl radicals (LOO˙) and to terminate the lipid peroxidation chain reactions (Equation (2))
[55][79], it can be seen that the mechanism of action is much more complex, as the antioxidants can act at successive steps of initiation, propagation, and chain termination of the oxidative radical process
[56][80].
OO˙ + α-tocopherol–OH → LOOH + α-tocopherol–O˙ (2)
It can be explained that there is a close correlation between the structure of antioxidants and their mode of action, determined by factors as follows
[62][63][84,85]:
86].
Table 1.
Inhibitors of lipid oxidation reactions.
-
The antioxidant effect increases proportionally with the length of the chain.
-
-
Alkylation in the meta position is less effective.
-
The esterification of the hydroxyl groups which causes a total disappearance of the antioxidant activity.
A classification of antioxidants according to their mode of action is presented in
Table 1 [61][64][83,
Glutathione peroxidase (GSHPx), catalase (CAT), and superoxide dismutase (SOD)
(mentioned in Figure 1) act as the first-line defense antioxidants, as their importance is especially related to superoxide anion radical (*O
2) which is perpetually generated in normal body metabolism, particularly through the mitochondrial energy production pathway (MEPP) and their fundamental role in preventing oxidative stress and the cellular damage
[5][55][60][31,79,82].
Glutathione is a nonenzymatic antioxidant that is found in most cells, and tissues of plants and animals, and in humans, the highest levels are in the liver, lens, pancreas, spleen, and kidney
[65][66][87,88]. It is mainly synthesized by the body
[66][88], and it can increase the level of cytotoxic T cells in lymphocytes and neutralize free radicals
[67][68][89,90]. Given that glutathione has a tripeptide composition of cysteine, glutamate, and glycine, it has an active thiol (SH−) within the cysteine structure
[6][8][32,34]. In the cell, >98% of glutathione is found in the reduced thiol form (GSH)
[5][66][31,88], but due to the cysteine residues that can be easily oxidized nonenzymatically by various electrophilic substances (free radicals, reactive oxygen, and nitrogen species), it is also present in the oxidized form as glutathione disulfide (GSSG) or glutathione peroxidase
[5][66][31,88]. After synthesis, it is distributed to intracellular compartments and the extracellular space for use by other cells and tissues
[66][68][88,90]. The rate of GSH synthesis is largely controlled by the degree of expression and catalytic activity of the enzyme γ-glutamyl-cysteine synthetase (GCS) and the cellular availability of cysteine
[5][60][66][67][68][31,82,88,89,90]. Oxidative stress, inflammatory cytokines, cancer, chemotherapy, ionizing radiation, heat shock, inhibition of GCS activity, GSH depletion, GSH conjugation, heavy metals, antioxidants, and insulin increase γ-glutamyl-cysteine synthase transcription or activity in a wide variety of cells
[69][70][91,92]. In contrast, protein deficiency, dexamethasone, erythropoietin, TNF-β (tumor necrosis factor), hyperglycemia, and GCS phosphorylation decrease GCS transcription or activity
[69][71][72][91,93,94]. The glutathione system also represents a “capture system” for peroxides from water metabolism and lipid peroxides permanently formed in the cell, metabolizing them with the formation of water and oxygen
[5][66][67][68][31,88,89,90]. It provides important protection for the mitochondrial and cell membrane against the harmful effects of reactive oxygen species (oxidative stress)
[5][31], protects the tertiary structure of proteins, and activates the transport of amino acids through the cell membrane
[5][73][31,95]. The cellular level of glutathione is stimulated by alpha lipoic acid, glutamine, colostrum, selenium, and vitamins C, B6, and B2, and the effectiveness of vitamins C, E, and coenzyme Q10 depends on the level of glutathione in the body
[74][96]. Food sources rich in GSH are generally green leafy vegetables, such as spinach, parsley, and broccoli. However, glutathione from food is only partially absorbed, being mostly hydrolyzed by peptidases
[74][75][76][96,97,98]. However, the diet plays an important role in the exogenous intake of glutathione by providing important cofactors, such as Se, Mn, Zn, and S-containing amino acids. GSH has a dual role in our health and pathology as an antioxidant and in the detoxification of certain xenobiotics
[74][75][76][96,97,98].
Catalases. While GSHPxs are cytosolic residents, catalases are mainly found in peroxisomes, in the liver, and erythrocytes, but some catalases are found in all tissues
[60][77][82,99], being the first characterized antioxidant enzymes
[66][88] and being one of the crucial antioxidant enzymes that mitigate oxidative stress to a considerable extent by destroying cellular hydrogen peroxide to produce water and oxygen by using either iron or manganese as a cofactor
[5][78][31,100]. Basically, they are present in almost all living tissues that utilize oxygen
[5][31]. Due to its chemical structure of four subunits, each containing a heme group and a molecule of NADPH, catalase basically catalyzes the conversion of hydrogen peroxide to water and oxygen
[60][82], while superoxide dismutase (one of the most potent intracellular enzymatic antioxidants) catalyzes the conversion of superoxide anions to dioxygen and hydrogen peroxide
[60][82]. Hence, all three, catalase, glutathione peroxidase, and superoxide dismutase, are functionally interconnected due to the hydrogen peroxide (H
2O
2), which is produced as a result of the reaction catalyzed by SOD, H
2O
2 being the substrate of both CAT and GSHPx
[65][87]. Deficiency or malfunction of catalase causes aging disorders and pathogenesis of degenerative diseases, such as diabetes mellitus, hypertension, anemia, vitiligo, Alzheimer’s disease, Parkinson’s disease, bipolar disorder, cancer, schizophrenia, or even male infertility
[78][79][100,101].
Superoxide dismutase. Depending on its expressed activity, superoxide dismutase may act either as an antioxidant or as a prooxidant
[66][88] as exists in several isoforms, differing in the active metal center, amino acid composition, cofactors, and other properties
[60][82], and neutralizes superoxide ions by going through successive oxidative and reductive cycles of transition metal ions at its active site
[60][82]. In humans, three forms of SOD are present: cytosolic Cu, Zn-SOD (consisting of a dinuclear metal cluster with copper and zinc ions, which catalyzes the dismutation of the superoxide anion to oxygen and water), the mitochondrial Mn-SOD (a homotetramer that includes one manganese atom per subunit, which partitions the superoxide anion), and the extracellular superoxide dismutase containing copper and zinc (a tetrameric secretary glycoprotein having a high affinity for certain glycosaminoglycans)
[60][80][81][82,102,103]. In contrast with the fact that superoxide dismutase is indispensable to cellular health, that is protecting body cells from oxidative stress, and that helps in the process of aging or cell death, superoxide dismutase enzyme deficiency is quite common, and more than this, levels of superoxide dismutase decline with age, whereas free radical formation increases
[5][31]. As a result, plant sources of SOD and SOD supplementation became of interest for health enhancement
[81][82][103,104]. It has been reported that a considerable and adequate daily SOD supplementation protects the immune system and significantly reduces the chances of degenerative diseases and aging pathogenesis, and there are several natural resources that can assure the daily intake of SOD, such as cabbage, Brussels sprouts, wheat grass, barley grass, or broccoli
[5][31].
If the first-line antioxidants act to suppress or prevent the formation of free radicals or reactive species in cells being very fast in neutralizing molecules with the potential of developing into a free radical or neutralizing any free radical with the ability to induce the production of other radicals
[5][31], the second-line defense antioxidants (scavenging antioxidants) are neutralizing or scavenging free radicals by donating an electron to them, becoming free radicals themselves but of lesser damaging effects
[5][60][31,82]. These are mainly represented by hydrophilic antioxidants, such as ascorbic acid, uric acid, and glutathione, and by lipophilic antioxidants, such as alpha-tocopherol (vitamin E) and ubiquinol
[5][60][31,82].
After free radical damage has occurred, a third category of antioxidants (de novo enzymes), such as polymerases, glycosylases, nucleases, proteinases, proteases, and peptidases, are acting towards repairing the damage caused to biomolecules and reconstitute the damaged cell membrane
[5][31], while a fourth-line defense antioxidants can prevent the formation or reaction of free radicals
[5][60][31,82].