Active pharmaceutical ingredients (APIs) can be defined as those biological compounds used for the detection, prevention, and treatment of different types of diseases. Asymmetric oxidation processes have constituted a valuable tool for the synthesis of APIs, especially for the preparation of optically active sulfoxides, compounds with interesting biological properties. Biocatalyzed reactions usually occur with high efficiency, excellent selectivity, good yields, environmental sustainability, and lower costs, which make them more attractive from an industrial perspective. However, it must be taken into account that these procedures also present some drawbacks, such as the (relatively) high substrate specificity of biocatalysts or the low substrate loadings required due to their generally low solubility in water.
- active pharmaceutical ingredients
- oxidations
- biocatalysis
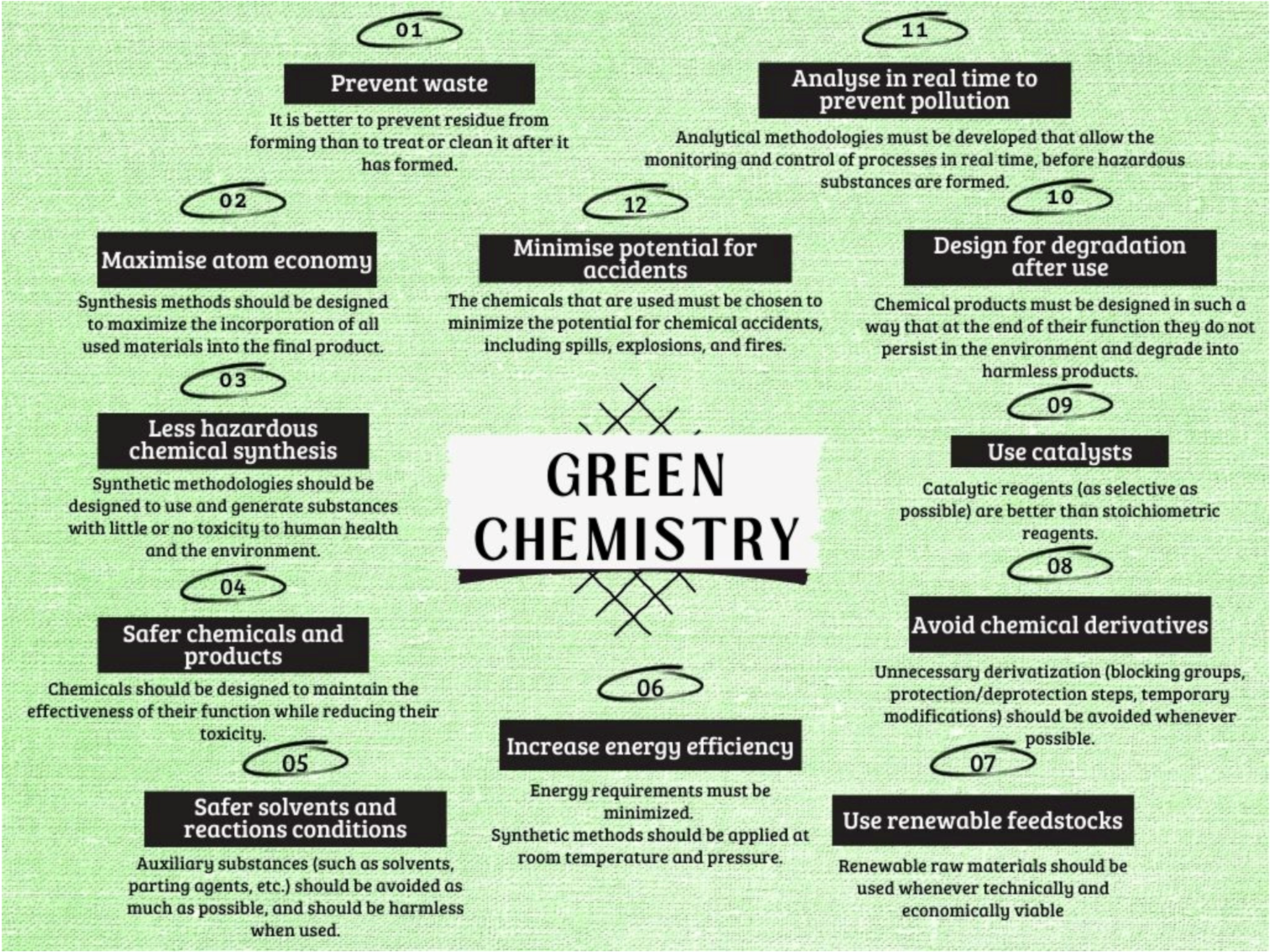
- Green Chemistry
1. Introduction
2. Biocatalytic Approaches for the Oxidative Preparation of APIs and Precursors
There are different types of catalysts employed in chemical synthesis, but enzymes in different preparations (well as free biocatalysts, cells free extracts, or whole cells systems) have attracted great interest since the last years of the past century [26,27,28,29][22][23][24][25]. In the mid-1980s, the development of cloning and overexpression techniques, the advances in immobilization procedures, and the application of enzymes in non-aqueous systems have allowed the development of several enzymatic methodologies for the synthesis of high added-value compounds, including the preparation of APIs [30,31,32,33,34][26][27][28][29][30]. Biocatalyzed reactions usually occur with high efficiency, excellent selectivity, good yields, environmental sustainability, and lower costs, which make them more attractive from an industrial perspective. However, it must be taken into account that these procedures also present some drawbacks, such as the (relatively) high substrate specificity of biocatalysts or the low substrate loadings required due to their generally low solubility in water. Oxidations carried out by biological systems present several advantages regarding classical methodologies [35,36][31][32]. Thus, biodegradable, non-toxic, and non-hazard catalysts are employed, whereas mild reaction conditions and molecular oxygen or hydrogen peroxide as mild oxidants are used. Oxidoreductases (E.C. 1.x.x.x) can perform the biocatalytic oxidation of different functional groups, including alcohols, amines, carbonyl compounds, and heteroatoms. This class of enzymes comprises different groups of biocatalysts, including dehydrogenases, oxidases, peroxidases, and oxygenases (both mono- and di-), presenting different properties and catalytic activities.2.1. Oxidations Catalyzed by Monooxygenases
Monooxygenases can catalyze the insertion of one atom from molecular oxygen into different molecules, whereas the other oxygen atom is released as water [37][33]. Among these enzymes, Baeyer-Villiger monooxygenases (BVMOs) are monooxygenases that contain flavin as prosthetic group and are able to catalyze the Baeyer-Villiger reaction and boron atom oxidation, as well as the oxygenation of different heteroatoms (sulfur, nitrogen, or phosphorous). BVMOs require nicotinamides (generally NADPH) as electron source for carrying out their activity. Due to its high price and instability, this cofactor must be recycled through a secondary enzymatic system as formate dehydrogenase (FDH), glucose or glucose-6-phosphate dehydrogenase (GDH or G6PDH), phosphite dehydrogenase (PTDH), or a ketoreductase (KRED). BVMOs have been employed for the preparation of optically active sulfoxides from prochiral sulfides in oxidative processes under mild and environmentally friendly conditions [38,39,40][34][35][36]. One example has been recently shown in the BVMO-catalyzed sulfoxidation step in the synthesis of AZD6738 (3), a candidate drug for the treatment of colon and hematological cancers [41][37]. This drug presents a chiral sulfoximine moiety in its structure, which is obtained from sulfoxide (R,R)–2. The initial synthesis of 2 from its prochiral sulfide 1 is carried out by employing m-chloroperbenzoic acid, which requires a chromatographic separation of the diasteromers obtained, yielding 50% of the desired sulfoxide as the highest yield. The application of the Kagan procedure in the presence of a titanium catalyst, afforded 2 in low yields, with degradation products. For these reasons, the biocatalytic approach for the oxidation of 1 was applied [42][38]. Reaction optimization was carried out using the design of experiments (DOE), analyzing the effect of substrate and enzyme concentrations, cosolvent charge, the type of buffer, and enzyme type. After analyzing more than 100 BVMOs, the best results were obtained with BVMO P1/D08 from the company Codexis in triethanolamine (TEA) buffer at pH 9.0, obtaining a 94% conversion starting from a sulfide concentration of 40 g/L and a BVMO loading of 24% weight (Scheme 3). The reaction was scaled up to the kilogram scale, analyzing all the parameters that could affect the process. Thus, an efficient agitation (500 rpm) and gas-liquid mass transfer (stream of air to supply O2 to the reaction) were required, as well as successive additions of iso-propanol (IPA), which was employed as cosolvent and cosubstrate to regenerate the cofactor NADPH in combination with the KRED CDX-019 to offset evaporation. Enzyme loading could be optimized up to 5% weight, achieving a 77% isolated yield after a one-day reaction. A further development in the biocatalyzed process was performed by carrying out the oxidations at higher scale (60 kg batches), leading to a 74% yield of the chiral sulfoxide with >99% enantiomeric excess (ee), which improves to a great extent the precedent chemical-synthesis protocols and increases the sustainability of the process.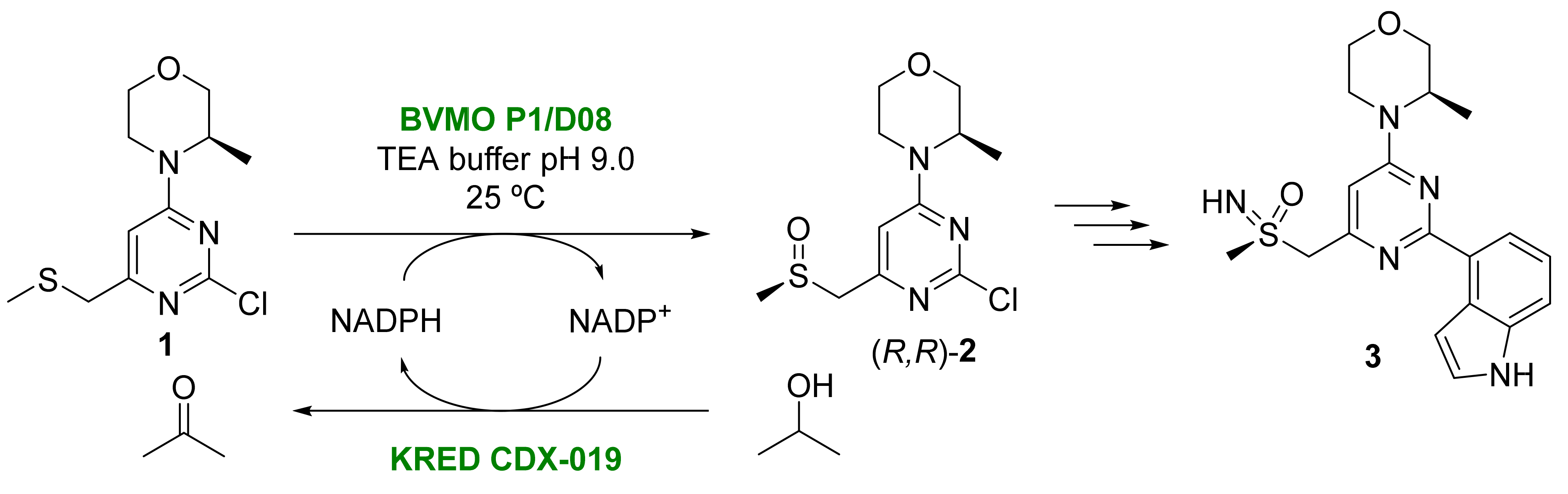
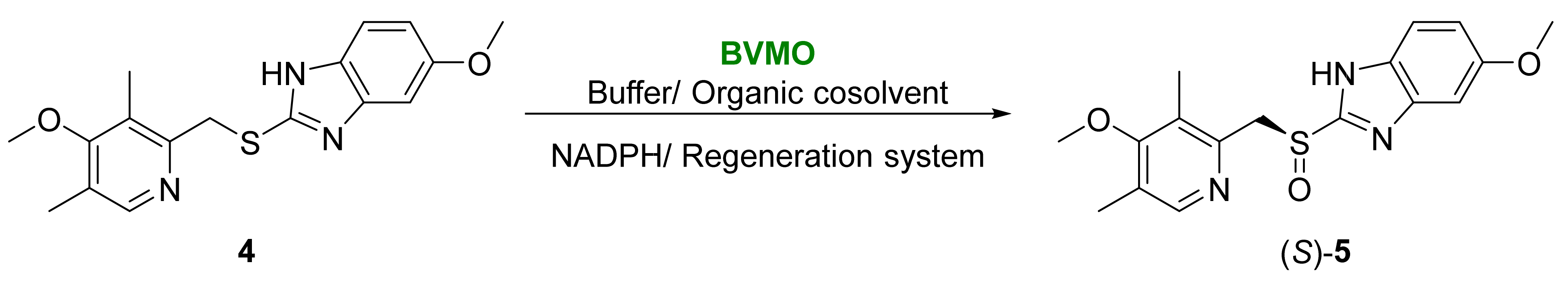
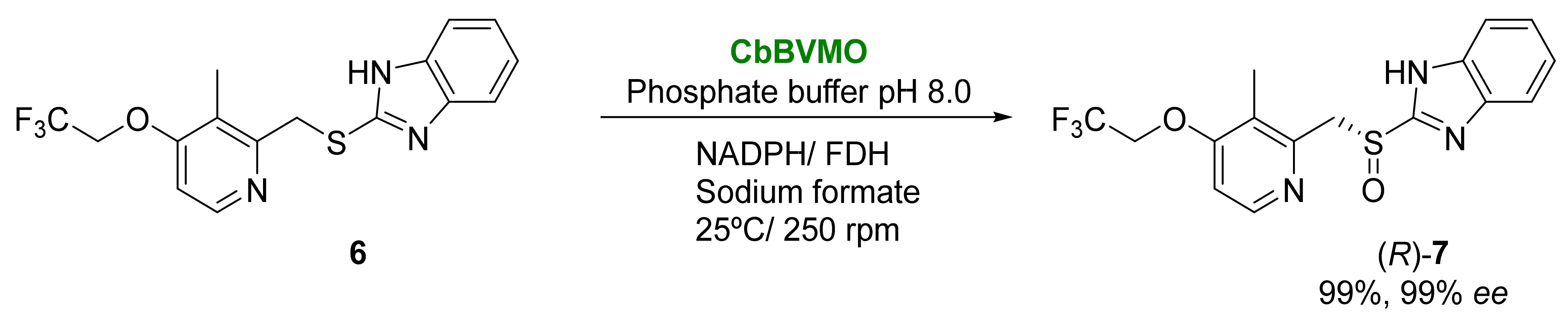
2.2. Other Biocatalyzed Oxidations
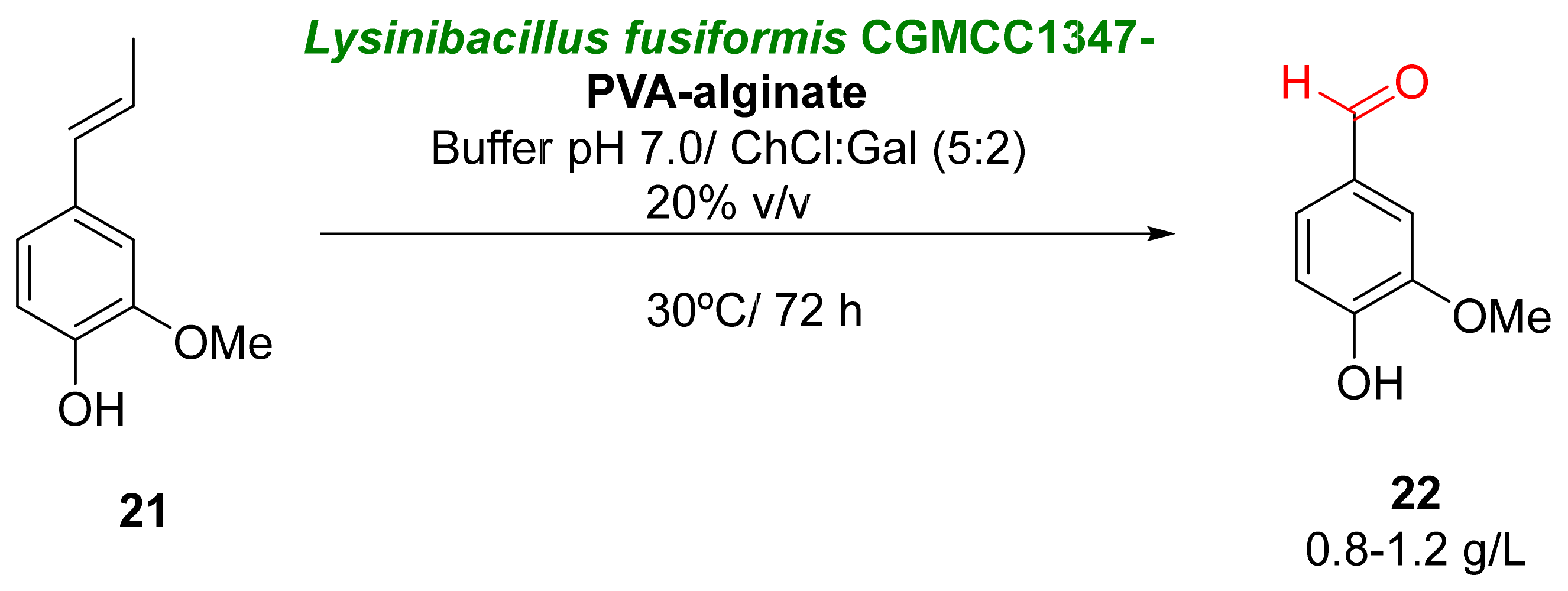
Islatravir (17) is a nucleoside analogue with high anti-HIV activity [60][56]. Several synthetic routes have been proposed for its preparation, but most of them require several steps with multiple protection/deprotection procedures. The biocatalytic approach proposed for the synthesis of 17 reduced the number of steps, making it possible to achieve the final product using a nine-enzyme system when starting from ethynyl glycerol 15 (Scheme 7) [61][57]. The initial step of this process consists in the selective oxidation of 15 to an aldehyde 16 catalyzed by a galactose oxidase from Fusarium graminearum. Oxidases are a class of oxidoreductases able to catalyze different oxidative reactions by employing molecular oxygen at mild reaction conditions [62,63,64][58][59][60]. The substrate oxidation and the reduction of molecular oxygen occur in the same active site, forming hydrogen peroxide as a byproduct and not requiring any cofactor for their activity. These enzymes have been employed in different types of oxidative procedures. Initial experiments were carried out with a galactose oxidase variant, which catalyzed the formation of the undesired (S)-enantiomer of compound 16 with moderate conversion, so several rounds of evolution were required to obtain mutants with improved activity, lower product inhibition, and a reversal on the enantioselectivity by mutating the positions W290 and F464. Oxidations were carried out in the presence of two additional enzymes: catalase, which was used to eliminate the hydrogen peroxide formed in the medium, and peroxidase, used to maintain the proper copper-oxidation state. Once the reaction conditions were optimized, it was possible to achieve (R)-16 with 97% enantiomeric excess and a high conversion.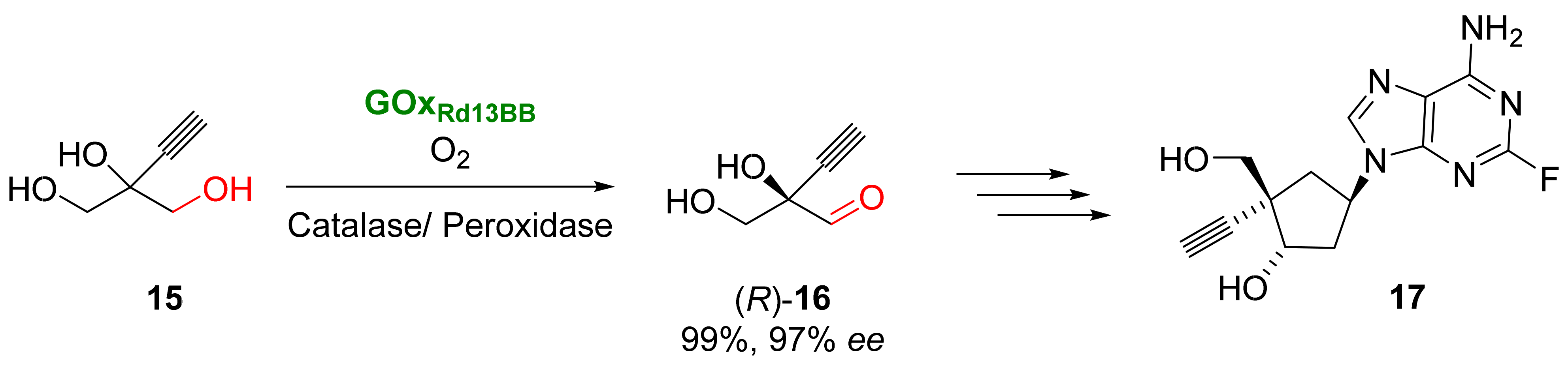
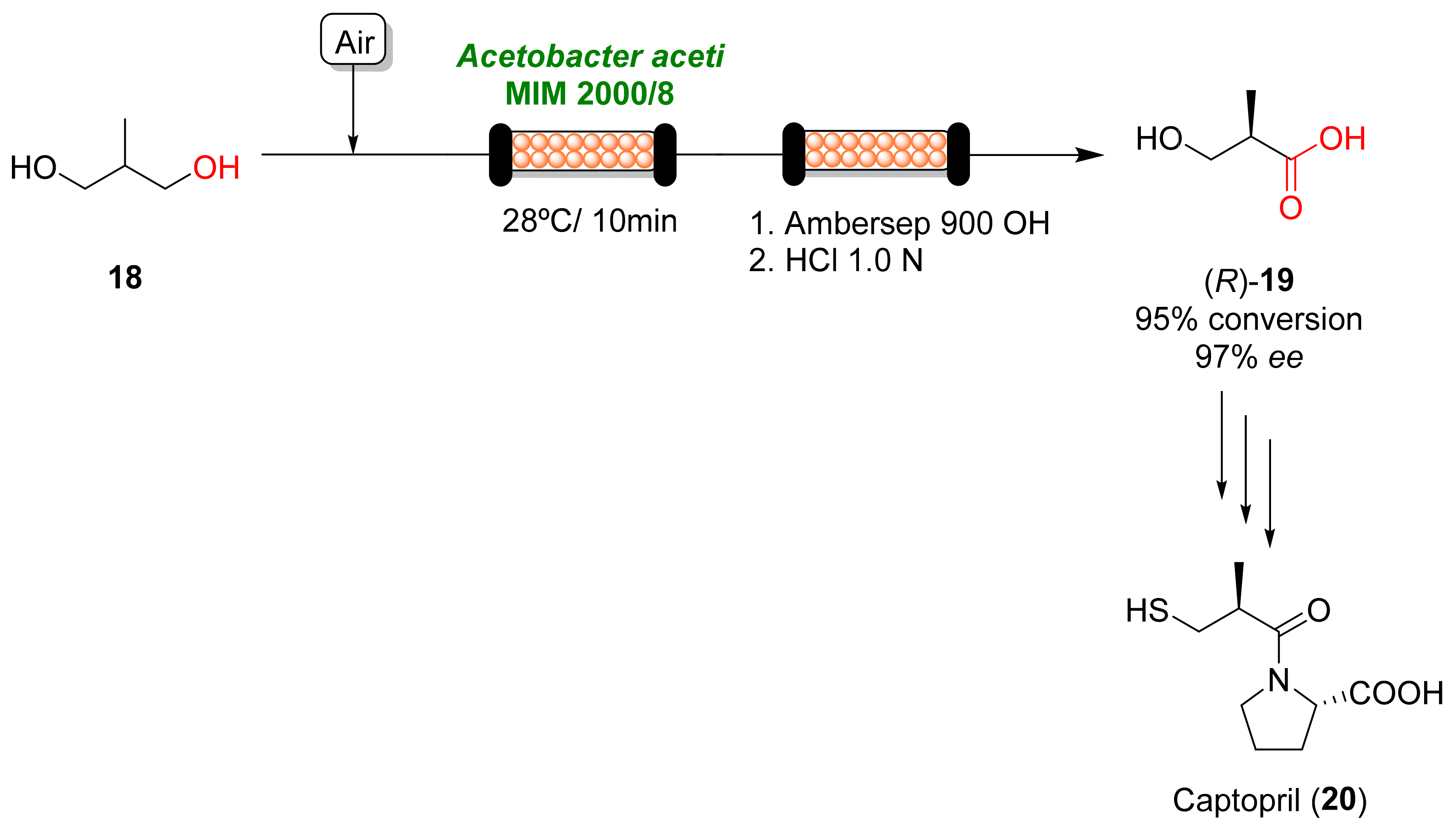
References
- U.S. Food & Drug Administration Glossary of Terms. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms (accessed on 22 January 2023).
- Park, J.; Kelly, M.A.; Kang, J.X.; Seemakurti, S.S.; Ramirez, J.L.; Hatzell, M.C.; Sievers, C.; Bommarius, A.S. Production of active pharmaceutical ingredients (APIs) from lignin-derived phenol and catechol. Green Chem. 2021, 23, 7488–7498.
- Horvath, I.T.; Anastas, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173.
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312.
- Sheldon, R.A. Fundamentals in Green Chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451.
- Anastas, P.T.; Williamson, T.C. Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes; Oxford University Press: Oxford, UK, 1998.
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998.
- United Nation Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 22 January 2023).
- Campos, K.R.; Coleman, P.J.; Alvárez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805.
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.K.; Handa, S. A glimpse into Green Chemistry practices in the pharmaceutical industry. ChemSusChem 2020, 13, 2859–2875.
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710.
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137.
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process Res. Dev. 2013, 17, 1517–1525.
- Plechkova, N.V.; Seddon, K.R. Application of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150.
- Liu, Y.; Friesen, J.B.; Lankin, J.B.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Miele, M.; Pillari, V.; Pace, V.; Alcántara, A.R.; de Gonzalo, G. Application of biobased solvents in asymmetric catalysis. Molecules 2022, 27, 6701.
- Miele, M.; Ielo, L.; Pillari, V.; Fernández, M.; Alcántara, A.R.; Pace, V. Biomass-Derived Solvents in Sustainable Organic Synthesis: Tools and Strategies; Protti, S., Palmieri, A., Eds.; Royal Society of Chemistry: Croydon, UK, 2022; pp. 239–279.
- Stuart, N.J.; Sanders, A.S. Phenyl Propionic Acids. US Patent 3385886, 2 February 1961.
- Andraos, J. Designing a green organic chemistry lecture course. In Green Organic Chemistry in Lecture and Laboratory; Dicks, A.P., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 2; pp. 29–68.
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic conversions in water. Part 5: Carbonylation of 1-(4-isobutylphenyl)- ethanol to ibuprofen catalysed by water-soluble palladium-phosphine complexes in a two-phase system. J. Chem. Technol. Biotechnol. 1997, 70, 83–91.
- Trost, B.M. Atom Economy: A challenge for organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 259–281.
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 108, 801–838.
- Domínguez de María, P.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as useful tool in asymmetric synthesis: An assessment of recently granted patents (2014–2019). Catalysts 2019, 9, 802.
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119.
- Domínguez de María, P. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514.
- Patel, R.N. Biocatalysis for synthesis of pharmaceuticals. Bioorg. Med. Chem. 2018, 26, 1252–1274.
- Simíc, S.; Zukíc, E.; Schmermund, L.; Faber, K.; Winkler, C.K.; Kroutil, W. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods. Chem. Rev. 2022, 122, 1052–1126.
- Rossini, G.; Robescu, M.S.; Licastro, E.; Tedesco, C.; Martello, I.; Maffei, L.; Vincenti, G.; Bavaro, T.; Collina, S. Biocatalysis: A smart and green tool for the preparation of chiral drugs. Chirality 2022, 34, 1403–1418.
- De Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P.; Sánchez-Montero, J.M. Biocatalysis for the asymmetric synthesis of Active Pharmaceutical Ingredients (APIs): This time is for real. Expert Opin. Drug Discover. 2022, 17, 1159–1171.
- Zawodny, W.; Montgomery, S.L. Evolving new chemistry: Biocatalysis for the synthesis of amine-containing pharmaceuticals. Catalysts 2022, 12, 595.
- Colonna, S.; Del Sordo, S.; Gaggero, N.; Carrea, G.; Pasta, P. Enzyme-mediated catalytic asymmetric oxidations. Heteroat. Chem. 2002, 13, 467–473.
- Dong, J.J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261.
- Torres-Pazmiño, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects, and biotechnological applications. J. Biotechnol. 2010, 146, 9–24.
- Leisch, H.; Morley, K.; Lau, P.C.K. Baeyer-Villiger monooxygenases: More than just green chemistry. Chem. Rev. 2011, 111, 4165–4222.
- Fürst, M.J.L.J.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer–Villiger monooxygenases: Tunable oxidative biocatalysts. ACS Catal. 2019, 9, 11207–11241.
- De Gonzalo, G.; Alcántara, A.R. Multienzymatic processes involving Baeyer–Villiger monooxygenases. Catalysts 2021, 11, 605.
- Foote, K.M.; Nissink, J.W.M.; Turner, P. Morpholinio Pyrimidines and Their Use in Therapy. Patent WO2011154737A, 11 June 2011.
- Goundry, W.R.F.; Adams, B.; Benson, H.; Demeritt, J.; McKown, S.; Mullholland, K.; Robertson, A.; Siedlecki, P.; Tomlin, P.; Vare, K. Development and scale-up of a biocatalytic process to form a chiral sulfoxide. Org. Process Res. Develop. 2017, 21, 107–113.
- Lindberg, P.; Brändström, A.; Wallmark, B.; Mattsson, H.; Rikner, L.; Hoffman, K.-J. Omeprazole: The first proton pump inhibitor. Med. Res. Rev. 1990, 10, 1–54.
- Baker, D.E. Esomeprazole magnesium (Nexium). Rev. Gastroenterol. Disord. 2001, 1, 32–41.
- Cotton, H.; Elebring, T.; Larsson, M.; Li, L.; Sörensen, H.; von Unge, S. Asymmetric synthesis of esomeprazole. Tetrahedron: Asymmetry 2000, 11, 3819–3825.
- Bong, Y.K.; Song, S.; Nazor, J.; Vogel, M.; Widegren, M.; Smith, D.; Collier, S.J.; Wilson, R.; Palanivel, S.M.; Narayanaswamy, K.; et al. Baeyer-Villiger monooxygenase-mediated synthesis of esomeprazole as an alternative for Kagan sulfoxidation. J. Org. Chem. 2018, 83, 7453–7458.
- Stewart, J.D. Cyclohexanone monooxygenase: A useful reagent for asymmetric Baeyer-Villiger reactions. Curr. Org. Chem. 1998, 2, 195–216.
- Zhang, Y.; Wu, Y.-Q.; Xu, N.; Zhao, Q.; Yu, H.-L.; Xu, J.-H. Engineering of cyclohexanone monooxygenase for the enantioselective synthesis of (S)-omeprazole. ACS Sustain. Chem. Eng. 2019, 7, 7218–7226.
- Xu, N.; Zhu, J.; Wu, Y.-Q.; Zhang, Y.; Xia, J.-Y.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L. Enzymatic preparation of the chiral (S)-sulfoxide drug esomeprazole at pilot-scale levels. Org. Process Res. Develop. 2020, 24, 1124–1130.
- Zhang, Y.; Liu, F.; Xu, N.; Wu, Y.-Q.; Zheng, Y.C.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L.; Xu, J.-H. Discovery of two native Baeyer-Villiger monooxygenases for asymmetric synthesis of bulky chiral sulfoxides. Appl. Environ. Microbiol. 2018, 84, e00638-18.
- Liu, F.; Shou, C.; Geng, Q.; Zhao, C.; Xu, J.; Yu, H. A Baeyer-Villiger monooxygenase from Cupriavidus basilensis catalyzes asymmetric synthesis of (R)-lansoprazole and other pharmaco-sulfoxides. Appl. Microbiol. Biotechnol. 2021, 105, 3169–3180.
- Urlacher, V.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic applications. Trends Biotechnol. 2012, 30, 26–36.
- Zhang, X.; Peng, Y.; Zhao, J.; Li, Q.; Yu, X.; Acevedo-Rocha, C.G.; Li, A. Bacterial cytochrome P450-catalyzed regio and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresur. Bioprocess. 2020, 7, 2.
- Nahri, L.O.; Fulco, A.J. Characterization of a catalytically self-sufficient 119,000 dalton cytochrome P450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1986, 26, 7160–7169.
- Acevedo-Rocha, C.G.; Gamble, C.; Lonsdale, R.; Li, A.; Nett, N.; Hoebenreich, S.; Deege, A. P450-catalyzed regio- and diastereoselective steroid hydroxylation: Efficient directed evolution enabled by mutability landscaping. ACS Catal. 2018, 8, 3395–3410.
- Schmitz, D.; Janocha, S.; Kiss, F.M.; Bernhardt, R. CYP106A2—A versatile biocatalyst with high potential for biotechnological production of selectively hydroxylated steroid and terpenoid compounds. BBA-Proteins Proteom. 2018, 1866, 11–22.
- Nikolaus, J.; Nguyen, K.T.; Virus, C.; Riehm, J.L.; Hutter, M.; Bernhardt, R. Engineering of CYP106A2 for steroid 9α- and 6β-hydroxylation. Steroids 2017, 120, 41–48.
- Litzenburger, M.; Kern, F.; Khatri, Y.; Bernhardt, R. Conversions of tricyclic antidepressants and antipsychotics with selected P450s from Sorangium cellulosum So ce56. Drug Metab. Dispos. 2015, 43, 392–399.
- Litzenburger, M.; Bernhardt, R. CYP260B1 acts as 9α-hydroxylase for 11-deoxycorticosterone. Steroids 2017, 127, 40–45.
- Ohrui, H.; Kohgo, S.; Hayakawa, H.; Kodama, E.; Matsuoka, M.; Nakata, T.; Mitsuya, H. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: A nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucl. Nucl. Nucleic Acids 2007, 26, 1543–1546.
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259.
- Dijkman, W.P.; de Gonzalo, G.; Mattevi, A.; Fraaije, M.W. Flavoprotein oxidases: Classification and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5177–5188.
- Pickl, M.; Fuchs, M.; Glueck, S.M.; Faber, K. The substrate tolerance of alcohol oxidases. Appl. Microbiol. Biotechnol. 2015, 16, 6617–6642.
- Wahart, A.J.C.; Staniland, J.; Miller, G.J.; Cosgrove, S.C. Oxidase enzymes as sustainable oxidation catalysts. R. Soc. Open Sci. 2022, 9, 211572.
- Wiles, C.; Watts, P. Improving chemical synthesis using flow reactors. Expert Opin. Drug Discover. 2007, 2, 1487–1503.
- De Santis, P.; Meyer, L.-E.; Kara, S. The rise of continuous flow biocatalysis—Fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184.
- Ötvos, S.B.; Kappe, O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advance interemediates. Green Chem. 2021, 23, 6117–6138.
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990.
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491.
- Ondetti, M.A.; Cushman, D.W. Azetidine-2-carboxylic Acid Derivatives. US Patent 4046889, 6 September 1977.
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of captopril. ChemistryOpen 2017, 6, 668–673.
- Romano, A.; Gandolfi, R.; Nitti, P.; Rollini, M.; Molinari, F. Acetic acid bacetria as enantioselective biocatalysts. J. Mol. Catal. B: Enzym. 2002, 17, 235–240.
- Kaur, B.; Chakraborty, D. Biotechnological and molecular approaches for vanillin production: A review. Appl. Biochem. Biotechnol. 2013, 169, 1353–1372.
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722.
- Domínguez de María, P.; Guajardo, N.; Kara, S. Enzyme catalysis: In DES. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 257–272.
- Hasani, F.Z.I.M.; Amzazi, S.; Lavandera, I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules 2019, 24, 2190.
- Czeisler, C.A.; Walsh, J.K.; Roth, T.; Hughes, R.J.; Wright, K.P.; Kingsbury, L.; Arora, S.; Schwartz, J.R.L.; Niebler, G.E.; Dinges, D.F. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N. Engl. J. Med. 2005, 353, 476–486.
- Pliszka, A.G. Modafinil: A review and its potential use in the treatment of long COVID fatigue and neurocognitive deficits. Am. J. Psychiatry Res. J. 2022, 17, 5–7.
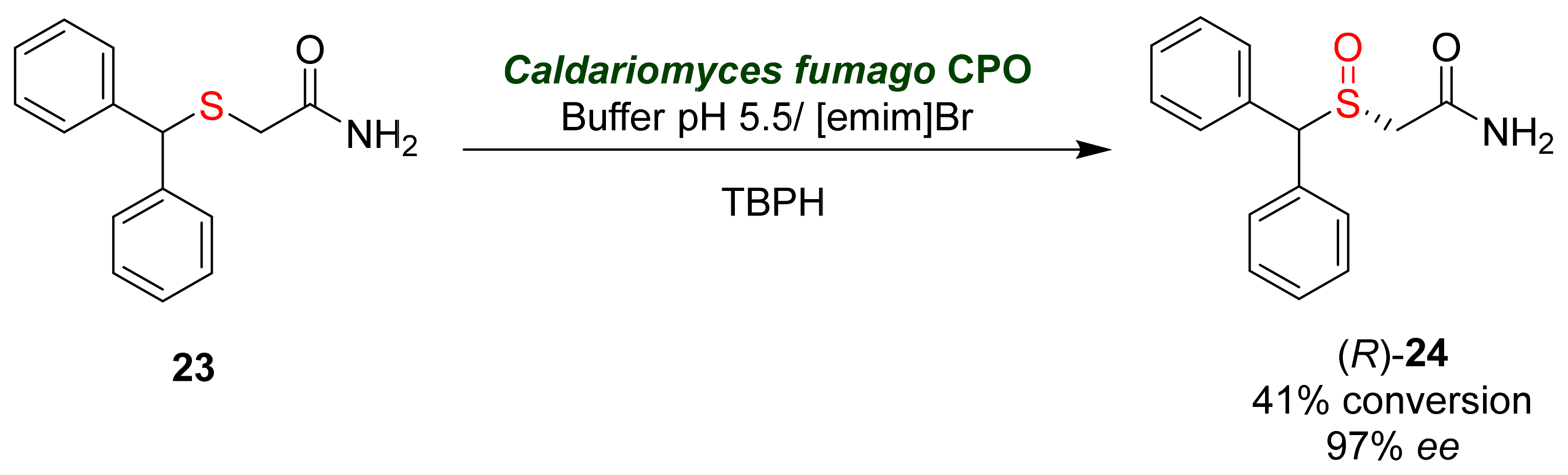
- Gao, F.; Wang, L.; Liu, Y.; Wang, S.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Enzymatic synthesis of (R)-modafinil by chloroperoxidase-catalyzed enantioselective sulfoxidation of 2-(diphenylmethylthio) acetamide. Biochem. Eng. J. 2015, 93, 243–249.
- Torres-Duarte, C.; Vazquez-Duhalt, R. Applications and prospective of peroxidase biocatalysis in the environmental field. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin, Germany, 2010.
- Itoh, T. Ionic Liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607.
