A severe and well-known threat to the environment, the non-biodegradability of plastics obliges different stakeholders to find legislative and technical solutions for producing valuable polymers which are biodegradable and also exhibit better characteristics for packaging products. Microorganisms are recognized as exciting sources for the production of biopolymers with applications in the food industry, package production, and several other fields. Ubiquitous organisms, lactic acid bacteria (LAB) are well studied for the production of exopolysaccharides (EPS), but much less as producers of polylactic acid (PLA) and polyhydroxyalkanoates (PHAs). Based on their good biodegradability feature, as well as the possibility to be obtained from cheap biomass, PLA and PHAs polymers currently receive increased attention from both research and industry.
- lactic acid bacteria
- polylactic acid
- polyhydroxyalkanoates
- exopolysaccharides
- food application
- food packaging
1. Introduction
2. Classification of Biopolymers Produced by Lactic Acid Bacteria
Biopolymers are linear or branched macromolecules made up of repeating units called monomers. Monomeric units are linked together by covalent bonds [11][12]. Depending on the nature of the repeating unit, biopolymers can be classified into groups like polysaccharides, glycolipids, lipopolysaccharides, proteins, etc. [8][12][13][14][8,13,14,15]. Biopolymers can be synthesized by plants, animals, and microorganisms [12][15][13,16]. Lactic acid bacteria (LAB) are capable of producing biopolymers with very different chemical structures, grouped into exopolysaccharides (EPSs), polyhydroxyalkanoates (PHAs), and polylactic acid (PLA) [8][13][14][8,14,15]. Based on their chemical composition, two groups of EPSs (Figure 1) can be identified: homopolysaccharides (HoPSs) formed from a single type of monosaccharidesand heteropolysaccharides (HePSs) formed from two or more types of monosaccharides [16][17][18][19][20][21][17,18,19,20,21,22].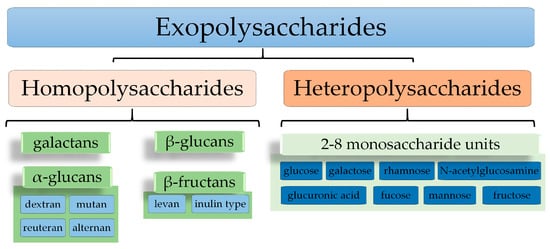
3. Biopolymers-Producing Lactic Acid Bacteria Strains
Lactic acid bacteria (LAB) have been empirically used, since ancient times, as starter cultures for the production of fermented foods and beverages and for preservation [8][43][44][8,44,45]. Due to their long history of safe use in human consumption [17][18], some LAB strains received the status Qualified Presumption of Safety (QPS) by the European Food and Safety Authority (EFSA) [45][46] or Generally Recognized as Safe (GRAS) by Food and Drug Administration (FDA) [46][47]. LAB comprise a heterogeneous group of genera [44][47][45,48] including Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Enterococcus, and Weissella, known for their wide industrial applications. Other representatives of LAB belong to Aerococcus, Alloiococcus, Carnobacterium, Dolosigranulum, Oenococcus, Tetragenococcus, and Vagococcus genera [48][49]. Members of the genera Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, and Oenococcus are considered GRAS [14][21][23][25][44][15,22,24,26,45]. LAB that belong to the genera Streptococcus and Enterococcus contain some opportunistic pathogens [49][50], and are not eligible for GRAS status. Safety concerns arising from their virulence factors and resistance to a variety of antibiotics [44][45] are associated with members of the genus Enterococcus; thus, they were not proposed for QPS status [50][51]. Important physiological properties are characteristics of all LAB, such as the capacity to ferment carbohydrates primarily into lactic acid via homo- or heterofermentative metabolism [44][49][45,50] and the inability to synthesize porphyrin groups (e.g., heme). LAB are Gram-positive, tolerant anaerobic, catalase-negative, cytochrome-deprived, non-spore-forming bacteria, with rod or coccus shape and with high tolerance at low pH [44][48][51][45,49,52]. Bacilli or cocci may appear as single or grouped cells, in tetrads and short or long chains [52][53]. These morphological characteristics emphasize the heterogeneity of the LAB group [44][45]. Lactic acid bacteria are intrinsically resistant to many antibiotics [49][50]. LAB are generally associated with nutritionally rich environments, because they are nutritionally demanding, with high requirements sources of carbon and nitrogen [52][53]. The optimum growth for LAB occurs at pH 5.5–5.8 [48][49], but they can also survive at pHs of around 5 and lower [8]. LAB are commonly found in vegetables, dairy and meat products, beverages, soil, and sewage, as well as in the gastrointestinal and genital tract of humans and higher animals [44][53][45,54]. Starter cultures of LAB with industrially important functionalities were developed in the last two decades, offering several technological, marketing, and health advantages, in order to meet the requirements of both producers and consumers. In industrial processes, LAB prove adaptation to stress conditions [8], such as acidic environment, temperature, salt concentration, etc. The optimal growth temperature, depending on the LAB genus and strain, ranges between 20 °C and 45 °C. Following certain metabolic pathways, LAB produce organic acids (mainly lactic acid, but also acetic acid), ethanol, antibacterial compounds (bacteriocins, hydrogen peroxide), vitamins, enzymes, aroma compounds, EPSs, etc. [17][54][18,55]. Citrate utilization results in diacetyl, acetoin, and 2,3-butanediol, whereas amino acid catabolism leads to volatile compounds and bioactive peptides [44][45]. Depending on the metabolites’ profile, LAB are used in different industrial applications. Production of bacteriocins, bioactive peptides, and antifungal compounds by some LAB is exploited to extend shelf life and enhance microbial safety of food, whereas compounds such as organic acids, volatile compounds, and exopolysaccharides contribute to the sensory and textural profile of some end-products [43][44][49][44,45,50]. Further, the LAB metabolic features allow for maintaining or even enhancement of the nutritional value of someraw materials [44][45]. Recently, LAB were used for the probiotic features of some strains, based on their ability to colonize the gastrointestinal tracts and proven competitiveness against pathogenic bacteria [49][50]. A constant increase in the market of functional foods was observed in the last years, with probiotics occupying an important segment, extending from dairy products to a wide range of non-dairy food products (such as vegetable-based, cereal-based, and sweet products).4. Polyesters from LAB
4.1. Polylactic Acid (PLA) Production Associated with LAB
Polylactic acid holds a leading position within the group of bio-degradable and bio-based plastics if rigid applications are discussed. However, different modification methods are applied to improve its performance in terms of heat stability and water barrier properties [55][80]. The polymerization process of LA into PLA, conducted since 1932, has been reviewed thoroughly in the literature. Two much-known methods of PLA production, namely direct poly-condensation (DPC) of lactic acid and ring-opening polymerization (ROP) are reconsidered nowadays aiming to eliminate the disadvantages of the chemical transformation of LA into PLA. From a chemical point of view, polylactic acid (PLA) is a polyester synthesized via lactic acid (LA). L or D isomers of lactic acid are produced through microbial fermentation of starch-rich agricultural products and then these monomers are chemically polymerized to obtain PLA. The monomers can be polymerized into pure poly-L-LA (PLLA), pure poly-D-LA (PDLA), or poly-D-LLA [56][81]. The physical properties of PLA are in a relationship with its enantiomer content [3]. Moreover, the morphological and mechanical characteristics of PLA are determined by the presence of different amounts of L-LA and D-LA monomers or oligomers [57][60]. Homopolymers of PLA are semicrystalline, whereas PLA heteropolymers are amorphous. Homofermentative methods are preferred because they lead to a higher yield of lactic acid with fewer by-products. This method uses Lactobacillus sp. such as Lactobacillus bulgaricus, L. delbrueckii, and L. leichmannii [58][82]. The three stages of PLA synthesis are well known, consisting of LA production (1), LA purification followed by cyclic lactides formation (2), and polycondensation of LA or ring-opening polymerization (ROP) of the cyclic lactides (3) [56][81]. Both polycondensation and the ROP method exhibit disadvantages. Thus, although the polycondensation is less expensive, it does not give a solvent-free high-molecular-weight PLA. The ROP route involves complicated and expensive purification steps and uses heavy metals as catalysts, as their residues are incompatible with applications of PLA for food contact surfaces [56][81]. Consequently, attention was focused on replacing the heavy metals catalysts with safe and environmentally acceptable alternatives and overcoming the challenge of the complete biosynthesis of PLA [59][83]. The biosynthesis of lactic acid is described in the following paragraphs, as the first stage of PLA production. Further, very recent efforts oriented towards designing the entire PLA production a bioprocess by developing alternatives to the DPC and ROP methods, namely, establishing a whole-cell biosynthetic system with recombinant microorganisms, are detailed.4.2. Polyhydroxyalkanoates (PHAs) Production by LAB
Polyhydroxyalkanoates (PHAs) represent a group of high-molecular-weight (about 105 Da) [60][64] biopolyesters, namely, polyhydroxybutyrate (PHB), polyhroxyvalerate (PHV), and derived polymers viz, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) [3] that are entirely degradable. The monomers of PHAs are always in the R(-) configuration due to the stereo-specificity of PHA synthases [60][64]. Accordingly, the PHAs exhibit several features similar to oil-derived plastics. PHAs are the only plastics exclusively produced by microorganisms [58][82], more specifically by bacterial anabolism [60][64]. PHAs are synthesized by bacteria as a stress response to the lack of essential inorganic nutrients (i.e., deprivation of nitrogen and phosphorus) and also in the situation of their growth phase [61][87]. Agro-industrial byproducts, e.g., milk and cheese whey can be subjected to microbial fermentation to obtain PHAs [60][64]. Diverse microorganisms produce and store PHAs as sources of carbon and adenosine-triphosphate (ATP). Species of Pseudomonas, Alcaligenes, and Bacillus are PHA-producing microbes. In more than 90 genera of microbial species documented, more than 150 different monomer constituents contain straight, branched, saturated, unsaturated, and aromatic structures in PHA [56][81]. Leuconostoc mesenteroides [62], Lactobacillus plantarum [13][14], and Lactobacillus bulgaricus [63][68] are PHAs producing lactic acid bacteria. Lactococcus, Lactobacillus, Pediococcus, and Streptococcus genera growing on MRS broth were reported as poly-β-hydroxybutyrate (PHB) producers [8], although the obtained yields were lower than the ones obtained in soil bacteria [63][68]. Recently, the mixed microbial cultures (MMCs), including LAB, growing on food wastes and other suitable biomasses are widely used for PHAs synthesis. In the last years, decreasing of PHAs production costs by developing alternative processes to pure culture fermentation processes was the focus of research works. Thus, two alternative processes were developed, namely the use of low-cost substrates coming from agro-industrial waste streams and that of MMCs [64][88] consisting of diverse bacterial genera. Engineering the microbial consortium by using the ecological selection principles was named recently eco-biotechnology. Activated sludge wastewaters, molasses, vegetable oil effluents, wheat and rice bran, and cheese wheyare only a few examples of substrates used to produce PHAs from MMC. A favorable impact on both PHA production and waste disposable costs could be reached by using waste materials as carbon sources for microbial-derived PHA production [65][89]. However, choosing the most suitable substrate is challenging, because the microorganisms’ metabolism and nutritional requirements must be carefully taken into account for high-yield PHA production. Moreover, the pre-treatments of the candidate carbon source and the choice of the PHA-producing strain are still hindered issues [65][89]. Thus, although milk whey is one of the most promising carbon-rich substrates, good PHA producers have displayed poor growth on lactose, whereas only a small part of microbial metabolism is directed to PHA production by good lactose utilizers [65][89]. The MMC PHA production requires lower operating costs because it does not need growth-medium sterilization prior to fermentation. Besides this advantage, MMCs are able to adapt to industrial waste complex substrates. Culture selection is the key to the effectiveness of MMC PHA production processes. Despite the above-mentioned advantages of using MMCs for PHAs production, more recently it was emphasized that better metabolic performances can be reached by using pure cultures of efficient PHA producers [60][64]. Better metabolic performances on whey with respect to PHA production, yet poorly explored, were attributed to pure cultures of lactic acid bacteria, evolving in the milk ecological niche [60][64]. From this starting point, the authors isolated from an MMC grown on dairy byproducts (cheese and scotta whey) PHA-producing strains, finding L. mesenteroides as one of the most active PHA-producing bacterial populations. Co-culture fermentation systems including LAB and Cupriavidus necator known for their ability to produce PHAs have also been reported [8]. Briefly, the lactic acid produced by LAB by conversion of carbohydrates is taken up by C. necator to producePHAs. Although recent literature is scarce concerning the development of co-culture fermentations for PHAs production, numerous related advantages are estimated to sustain future application of co-cultures, such as increased yield with improved control of product qualities and the possibility of utilizing secondary products, cheaper than glucose [8].5. Exopolysaccharides from LAB
Exopolysaccharides (EPSs) are polymeric carbohydrate molecules, namely extracellular polysaccharides that are either associated with the cell surface as capsules, called capsular exopolysaccharides (capsular EPSs), or secreted into the extracellular environment as slime, called slime exopolysaccharides (slime EPSs) [17][18][25][66][18,19,26,90]. EPSs’ role is to store energy and protect the bacterial cell against unfavorable environmental factors such as temperature, pH, osmotic pressure, desiccation, light, phagocytosis, bacteriocins, protozoa, and toxic compounds (toxic metal ions, sulfur dioxide, ethanol, and antibiotics) [23][25][67][24,26,91]. EPS production and secretion start during bacterial growth and stop in the stationary phase [25][68][26,92]. They are synthesized intracellularly and secreted outside the cell, or are produced extracellularly by enzymes secreted by lactic acid bacteria [21][22]. Bacterial EPSs have a wide range of industrial applications (i.e., food, medicine, pharmaceuticals, and cosmetics) depending on their physicochemical and structural properties. The costs of production are related to the costs of the carbon sources and the EPS yield. Thus, the bacterial EPSs entering the market are relatively limited. The first microbial EPS that was commercialized was dextran [69][93]. Identifying new bacteria producing EPSs at high yields and also with functional features led recently to much interest in lactic acid bacteria [70][94]. EPS yield is strain-specific and heavily influenced by the substrate used in terms of the nutritional and growing conditions. Food wastes (FWs) are seen as an excellent choice for EPSs production by LAB, both to minimize environmental contamination and also to generate economically relevant EPSs [70][94]. Many review articles discussed the production of the exopolysaccharide by synthetic LAB strains and their physical, chemical, and biological properties related to specific applications in the food industry and health [71][72][95,96]. Most EPS-producing LAB belong to the genera Lactobacillus, Streptococcus, Lactococcus, Leuconostoc, and Weissella. Approximately 30 species of lactobacilli have been reported to produce EPSs, especially L. casei, L. acidophilus, L. brevis, L. curvatus, L. delbrueckii subsp. bulgaricus, L. helveticus, L.rhamnosus, L. plantarum, L. johnsonii, etc. [73][74][97,98]. LAB may synthesize EPSs (heteropolysaccharides or homopolysaccharides) within an enormous structural diversity [75][76][77][99,100,101] that are differentiated by their monosaccharides’ composition, molecular mass, size, and structure [17][25][18,26]. Some possible physiological roles of EPSs are to help LAB in their survival [78][102] and to offer LAB protection from stress conditions (such as environmental pH, osmotic stress, lack of essential elements such as nitrogen, protection from bacteriophages, antibiotics, lysozymes, etc.) [51][76][79][52,100,103]. EPSs production by LAB occurs not only under growth-limiting conditions but also in the presence of excess available carbohydrates (i.e., sucrose) [79][103]. The formation of mucoid colonies in solid media and the increase in viscosity in liquid media is the basis of the detection of the presence of EPSs associated with bacterial cells [79][103]. EPSs are loosely attached to the cell or secreted to the environment [80][67]. EPSproduction is strain-dependent and is strongly affected by the processing conditions (i.e., carbon source and nutrients existing in the culture medium, pH and temperature, incubation time, etc. [17][71][18,95]). The monomer blocks are polymerized at the cell wall, and EPSs are either liberated into the medium (free EPSs) or remain attached to the bacteria (capsular EPSs). Some LAB strains produce both forms, others only free EPSs. According to the ropy character of the fermented milk, the free EPSs can be further classified [81][69]. Mentioned distinctively from EPSs [80][67], the capsular polysaccharides (CPS) are covalently bound to the cell surfaceand structurally can be of the HoPS or HePS type. According to these authors, EPS and capsular polysaccharide LAB producers are frequently belonging to the genera Lactobacillus, Leuconostoc, Streptococcus, Lactococccus, and Weissella. The greatest variety of structures was reported in lactobacilli [75][99]. EPSs exhibit a broad range of physic-chemical functionalities and applications [51][71][52,95]. Thus, microbial EPS are recognized as bio thickeners due to their stabilizing, emulsifying, viscosifying, or gelling capacity [25][26] and particularly contribute to the sensory and rheological properties of fermented foods, as well as to their stability [51][52]. Further, EPS confer unique properties to fermented food, properties that are generally beneficial to humans [78][79][102,103], which is why some of them fulfill the criteria considered for functional foods. Recently, EPS were considered functional postbiotic ingredients in fermented foods [51][52], due to their human health benefits, such as immuno-modulation, anti-oxidative, anti-inflammatory, anti-microbial, or microbiome modulators. The EPS production by probiotic LAB seemed to be responsible for their health effects, such as LAB persistence in the gut ecosystem [82][104]. However, despite the above-mentioned benefits of EPS produced by LAB, with the exception of homopolysaccharide dextran, until now, only the in situ application of EPS-producing LAB (i.e., as starter cultures) has been economically viable [51][52]. This is due to the low yield of EPS production by LAB (in comparison with other EPS-producing strains), the required steps of EPS purification, as well as production costs. It was suggested that yields should be in the range of 10–15 g/L for an economically feasible production of EPS to use as a food ingredient [23][24].6. Processing Methods of Biopolymers Produced by LAB
To improve the functional properties the biopolymers produced by LAB can be modified by phosphorylation, sulfonation, and acetylation. Phosphorylated EPSs and sulfated EPSs exhibit better superoxide and hydroxyl radical scavenging ability, respectively an increased antioxidant activity. Sulphonated EPSs have a stronger inhibitory effect on Gram-positive and Gram-negative pathogens. Acetylated groups confer EPSs more flexible, elastic, antioxidant, and thermo-reversible properties [15][16]. Improved properties are also obtained by combining two biopolymers. Thus, PLA with poly(3-hydroxybutyrate) (PHB) films demonstrated a good barrier to water vapor [83][117]. EPSs composed of manan (produced by Weissella confusa MD1) and EPSs composed of glucose, galactose, mannose, and arabinose (produced by Lact. fermentum S1) have significant activity against food-borne pathogens [84][118]. In order to protect food and extend shelf life, food packaging/edible coating must have a number of physical, chemical, and functional properties; namely, to provide a barrier for water vapor and oxygen, to be permeable to CO2, to have good mechanical properties [83][117], to present antioxidant and antimicrobial capacity [84][118], and to be flexible [85][119], transparent, and biodegradable [83][117]. In order toimprov their properties, the biopolymers secreted by LAB are subjected to various processing, as follows:-
for higher flexibility, plasticizers that have the ability to increase the mobility of biopolymer chains due to the reduction of intermolecular forces are added. Thus, to improve flexibility the kefiran films are plasticized with sorbitol, galactitol [85][119], glycerol, oleic acid, polyols, and sugars (glucose, galactose, sucrose) [83][117], and levan films, with glycerol [86][120].
-
enhanced mechanical properties can be achieved when composite films made of EPSs, lipids, and hydrocolloids are formed [84][118]. Moreover, EPSs combined with starch (corn starch, cassava starch) form films with improved mechanical and chemical properties [83][117], and nanocomposite films composed of starch/kefiran/ZnO [87][121] or levan and starch have increased tensile strength [84][118].
