Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Adriana Basile.
The biological effects of heavy metals have been studied in both animals and plants, ranging from oxidative stress to genotoxicity. Plants, above all metal-tolerant species, have evolved a wide spectrum of strategies to counteract exposure to toxic metal concentrations.
- antioxidant enzymes
- bryophyte
- heavy metals
- glutathione
- phytochelatins
- stress responses
- metal tolerance
1. Metal Tolerance and Accumulation of Heavy Metals in Bryophytes
Bryophytes lack an absorptive root system, have a cuticle endowed with high permeability and exhibit a pronounced cell-wall ion-exchange capacity, all of which enables them to efficiently absorb minerals across their entire body surface [23][1]. This property is a fundamental adaptive factor for most, if not all, bryophytes, but, at the same time, it results in high sensitivity to toxic chemical species present as contaminants in the environment. In the presence of the same elements and environmental conditions, bryophytes can exhibit different types of behaviour, with different biochemical mechanisms allowing them to tolerate high concentrations of heavy metal. Metal tolerance is the ability of a species to survive in environments where metal(loid)s contents are toxic for most other species [24][2]. It can be a constitutive property (genetically determined), or induced as a result of exposure to heavy metals, or mixed in nature [25][3]. For example, Basile et al. [11][4] found only the moss Funaria hygrometrica growing on the soil of lead and zinc mine dumps, which probably represented an ecotype with a high tolerance to such prohibitive environmental conditions [11][4]. In a subsequent study, Basile et al. [26][5] showed that spores of F. hygrometrica collected from a metal-polluted site developed in a normal protonemata, whilst those from an unpolluted site grew in an altered protonemata. Comparably, Jules and Shaw [27][6], observed that protonemata developed in vitro from samples of Ceratodon purpureus growing near a smelter were more tolerant to Zn, Cd and Pb than those from unpolluted sites. Constitutive metal tolerance is stable and unaffected by environmental conditions; induced metal tolerance, being a form of physiological acclimatization to the environment, persists as long as the specific stressors that led to its onset exist [28][7]. In most cases, tolerance appears to be genetically determined rather than induced, and selection for tolerance can be severe in highly contaminated habitats [28,29][7][8].
The metal tolerance in bryophytes has been investigated in several studies. Populations of the same species that colonize urban environments might have been selected for tolerance to Pb, as observed by Briggs et al. [30][9], comparing Marchantia polymorpha from unpolluted and polluted urban environments. Similarly, Wells and Brown, 1995 [31][10] compared two populations of Rhytidiadelphus squarrosu, one collected from an unpolluted site and the other from a zinc mine. Samples from a contaminated site showed a lower loss rate in photosynthetic activity than those from an unpolluted one. The existence of metal-tolerant ecotypes in bryophytes was observed in several other studies [32,33,34][11][12][13]. Patterns of heavy metal uptake and accumulations in bryophytes have been investigated both in mosses and liverworts. Basile et al. [35][14] characterized uptake and localization of Pb in Funaria hygrometrica. The results showed that Pb was accumulated preferentially in some parts of the gametophyte and in the lower parts of the sporophyte. On the other hand, no Pb was detected in the spores and capsule (i.e., upper part of the sporophyte). Basile et al. [11][4] confirmed the previous results analysing Pb and Zn content in F. hygrometrica collected from a mine-tailing site. The authors observed that Pb and Zn were mostly accumulated into the gametophytes (1000- to 2000-fold more) than in the sporophyte. A few years later, Carginale et al. [36][15] investigated the accumulation and localization of Cd in the liverwort Lunularia cruciata. The results showed that Pb accumulated mainly in the hyaline parenchima (i.e., non-photosynthetic tissue with large vacuoles) and was sequestered into vacuoles. These data suggest that bryophytes tend to avoid the accumulation of heavy metals in the reproductive structures (i.e., sporophyte, spores, and gemmae), and sequester them into the vacuole of the gametophyte cells.
2. Bryophytes’ Defences against Heavy Metals
Heavy metals, especially those which do not have a role in bryophytes’ physiology (e.g., Pb, Cd, Hg), cause harmful effects, starting from the cellular level, which may cause physiological impairments in the whole organism. This happens when the molecular machinery cannot manage the excess of heavy metal in the cytoplasm. Several studies have investigated the harmful effects of heavy metals in bryophytes. Some researchers have characterized the damage at the ultrastructural level. Basile et al. [12][16] and Esposito et al. [37][17] reported that metals such as Cd and Pb (Cd > Pb) cause severe alterations in the cell ultrastructure. The authors observed dose-dependent alterations: swollen chloroplasts, irregular thylakoids organization, increased plastoglobules, swollen mitochondria cristae; and cellular signs of senescence (i.e., multivesicular bodies). Similar alterations were observed by Choudhury and Panda [38[18][19],39], in the moss Taxithelium nepalense after Pb and As exposure. Other studies pointed out the fact that heavy metal uptake causes a decrease in chlorophyll content [40[20][21][22][23][24],41,42,43,44], and a decrease in photosynthetic activity [45,46,47,48][25][26][27][28]. These changes at the cellular level cause toxicity at the organism level due to alteration of the normal metabolism. In fact, some other investigations reported growth inhibition of bryophytes exposed to toxic metals such as Pb and Cd [43,49,50,51][23][29][30][31].
The metal tolerance in bryophytes could have an explanation in precise cellular responses such as the activation of certain enzymes and the synthesis of defence proteins.
As in other plant organisms, in bryophytes, the first barrier against heavy metal stress is mediated by the cell wall through chelation and immobilization via pectic compounds. Several studies have investigated the immobilization of heavy metals in the cell walls of bryophytes [11,35,36,52,53][4][14][15][32][33]. This passive mechanism reduces the amounts reaching young or reproductively affected parts and, at the cellular level, the amounts able to penetrate the cytoplasm and exert toxic effects. Heavy metals bind to the negative charges of cell-wall polysaccharides rich in carboxyl groups (homogalacturonans) and other functional groups (–OH and –SH), as well as proteins, phenolics, and amino acids [54,55][34][35]. This process mainly affects tissues that exhibit cell-wall modifications, such as hydroids, placental “transfer cells”, or hyaline parenchyma cells. This process seems to be increased by stress conditions. In fact, the break-up of cell membrane in dead or damaged cells may cause the freeing of more cation-binding sites, thus allowing a higher accumulation of metals in the cell wall of dead or damaged cells [56][36]; there is proof that bryophytes under heavy metal stress can rearrange the cell wall by thickening it and increasing the amount of low-esterified and unesterified homogalacturonan [57,58][37][38]. In general, these mechanisms aim to provide more binding sites for the immobilization of heavy metals in the cell wall.
The cell wall thus represents a passive barrier to prevent heavy metals from entering into and interacting with the cytoplasmic environment. However, heavy metals that enter the cytosol require a wide range of molecular responses to avoid harmful effects to cellular structures. Bryophytes have developed, like higher plants, a series of cellular responses to counteract heavy metal stresses that collectively take the name “fan response” (Figure 1). These cellular mechanisms include the chelation and compartmentalization of heavy metals, as well as the activation of non-enzymatic and enzymatic antioxidant defences to counteract the induced reactive-oxygen-species (ROS) production. Several studies have indicated that these mechanisms involve the synthesis of molecules capable of binding such ions (e.g., amino acids, citric acid, malic acid) [59[39][40],60], the modulation of the enzymatic antioxidant system (i.e., SOD, CAT, GPX, POX, etc.) [38,45,61[18][25][41][42][43][44],62,63,64], increase in the phenolic content [65][45], increase in lunularic acid synthesis [65][45], and increased synthesis of phytochelatins, glutathione and “heat shock protein” (HPS) [37,66,67[17][46][47][48][49][50],68,69,70], phenomena largely mediated by gene activation/repression (Figure 1).
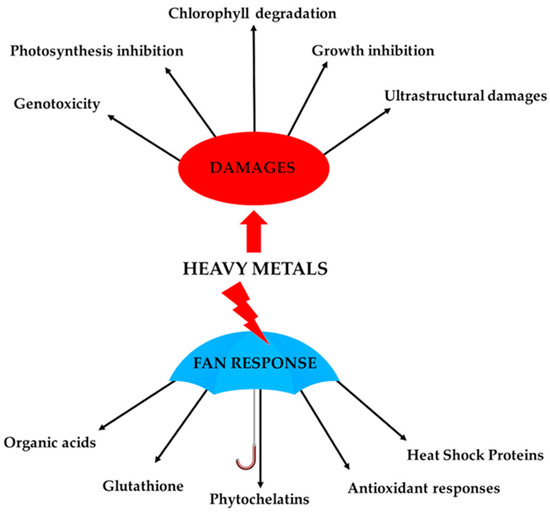
Figure 1.
Schematic figure of cellular responses and alterations caused by heavy metals in bryophytes.
References
- Bargagli, R. Trace Elements in Terrestrial Plants: An Ecophysiological Approach to Biomonitoring and Biorecovery; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-64551-1.
- Macnair, M.R.; Tilstone, G.H.; Smith, S.E. The Genetics of Metal Tolerance and Accumulation in Higher Plants; CRC Press: Boca Raton, FL, USA, 2020; pp. 235–250. ISBN 978-0-367-80314-8.
- Ernst, W.H.O. Evolution of Metal Tolerance in Higher Plants. For. Snow Landsc. Res. 2006, 80, 251–274.
- Basile, A.; Cogoni, A.E.; Bassi, P.; Fabrizi, E.; Sorbo, S.; Giordano, S.; Cobianchi, R.C. Accumulation of Pb and Zn in Gametophytes and Sporophytes of the Moss Funaria Hygrometrica (Funariales). Ann. Bot. 2001, 87, 537–543.
- Basile, A.; Sorbo, S.; Bassi, P.; Napolitano, E.; Cogoni, A.E.; Cobianchi, R.C. Effects of Heavy Metals on Protonemal Development and Ultrastructure in Populations of the Moss Funaria Hygrometrica Hedw. (Bryophyta) from a Mine and an Unpolluted Site. Fresenius Environ. Bull. 2008, 17, 1956–1963.
- Jules, E.S.; Shaw, A.J. Adaptation to Metal-Contaminated Soils in Populations of the Moss, Ceratodon Purpureus: Vegetative Growth and Reproductive Expression. Am. J. Bot. 1994, 81, 791–797.
- Boyd, R.S.; Martens, S.N. Nickel Hyperaccumulation by Thlaspi Montanum Var. Montanum (Brassicaceae): A Constitutive Trait. Am. J. Bot. 1998, 85, 259–265.
- Meharg, A.A. Integrated Tolerance Mechanisms: Constitutive and Adaptive Plant Responses to Elevated Metal Concentrations in the Environment. Plant Cell Environ. 1994, 17, 989–993.
- Briggs, D. Population Differentiation in Marchantia Polymorpha L. in Various Lead Pollution Levels. Nature 1972, 238, 166–167.
- Wells, J.M.; Brown, D.H. Cadmium Tolerance in a Metal-Contaminated Population of the Grassland Moss Rhytidiadelphus Squarrosus. Ann. Bot. 1995, 75, 21–29.
- Brown, D.H.; House, K.L. Evidence of a Copper-Tolerant Ecotype of the Hepatic Solenostoma Crenulatum. Ann. Bot. 1978, 42, 1383–1392.
- Shaw, J. Genetic Variation for Tolerance to Copper and Zinc within and among Populations of the Moss, Funaria Hygrometrica Hedw. New Phytol. 1988, 109, 211–222.
- Boquete, M.T.; Spagnuolo, V.; Fernández, J.Á.; Aboal, J.R.; Imperatore, I.; Giordano, S. Genetic Structuring of the Moss Pseudoscleropodium Purum Sampled at Different Distances from a Pollution Source. Ecotoxicology 2016, 25, 1812–1821.
- Basile, A.; Giordano, S.; Cafiero, G.; Spagnuolo, V.; Castaldo-Cobianchi, R. Tissue and Cell Localization of Experimentally-Supplied Lead in Funaria Hygrometrica Hedw. Using X-Ray SEM and TEM Microanalysis. J. Bryol. 1994, 18, 69–81.
- Carginale, V.; Sorbo, S.; Capasso, C.; Trinchella, F.; Cafiero, G.; Basile, A. Accumulation, Localisation, and Toxic Effects of Cadmium in the Liverwort Lunularia Cruciata. Protoplasma 2004, 223, 53–61.
- Basile, A.; Sorbo, S.; Conte, B.; Cobianchi, R.C.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, Accumulation, and Removal of Heavy Metals by Three Aquatic Macrophytes. Int. J. Phytoremediat. 2012, 14, 374–387.
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of Heavy Metals on Ultrastructure and HSP70S Induction in the Aquatic Moss Leptodictyum Riparium Hedw. Int. J. Phytoremediat. 2012, 14, 443–455.
- Choudhury, S.; Panda, S.K. Toxic Effects, Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. under Chromium and Lead Phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90.
- Choudhury, S.; Panda, S.K. Induction of Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. under Lead and Arsenic Phytotoxicity. Curr. Sci. 2004, 87, 342–348.
- Świsłowski, P.; Rajfur, M.; Wacławek, M. Influence of Heavy Metal Concentration on Chlorophyll Content in Mosses. Ecol. Chem. Eng. S 2020, 27, 591–601.
- Phaenark, C.; Niamsuthi, A.; Paejaroen, P.; Chunchob, S.; Cronberg, N.; Sawangproh, W. Comparative Toxicity of Heavy Metals Cd, Pb, and Zn to Three Acrocarpous Moss Species Using Chlorophyll Contents. Trends Sci. 2023, 20, 4287.
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of Heavy Metals (Copper, Zinc, and Lead) on the Chlorophyll Content of Some Mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421.
- Stanković, J.D.; Janković, S.; Lang, I.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. The Toxic Metal Stress in Two Mosses of Different Growth Forms under Axenic and Controlled Conditions. Bot. Serbica 2021, 45, 31–47.
- Tremper, A.H.; Agneta, M.; Burton, S.; Higgs, D.E.B. Field and Laboratory Exposures of Two Moss Species to Low Level Metal Pollution. J. Atmos. Chem. 2004, 49, 111–120.
- Maresca, V.; Bellini, E.; Landi, S.; Capasso, G.; Cianciullo, P.; Carraturo, F.; Pirintsos, S.; Sorbo, S.; Sanità di Toppi, L.; Esposito, S.; et al. Biological Responses to Heavy Metal Stress in the Moss Leptodictyum Riparium (Hedw.) Warnst. Ecotoxicol. Environ. Saf. 2022, 229, 113078.
- Brown, D.H.; Wells, J.M. Physiological Effects of Heavy Metals on the Moss Rhytidiadelphus Squarrosus. Ann. Bot. 1990, 66, 641–647.
- Chen, Y.-E.; Mao, H.-T.; Ma, J.; Wu, N.; Zhang, C.-M.; Su, Y.-Q.; Zhang, Z.-W.; Yuan, M.; Zhang, H.-Y.; Zeng, X.-Y.; et al. Biomonitoring Chromium III or VI Soluble Pollution by Moss Chlorophyll Fluorescence. Chemosphere 2018, 194, 220–228.
- Díaz, S.; Villares, R.; Vázquez, M.D.; Carballeira, A. Physiological Effects of Exposure to Arsenic, Mercury, Antimony and Selenium in the Aquatic Moss Fontinalis Antipyretica Hedw. Water Air Soil Pollut. 2013, 224, 1659.
- Gupta, A.; Chopra, R.N. Effect of Some Heavy Metals on Growth and Archegonial Formation in the Female Clones of Riccia Discolor (Hepaticae, Ricciaceae) Grown in Vitro. Fragm. Florist. Geobot. 1995, 40, 533–544.
- Sidhus, M.; Brown, D.H. A New Laboratory Technique for Studying the Effects of Heavy Metals on Bryophyte Growth. Ann. Bot. 1996, 78, 711–717.
- Sassmann, S.; Weidinger, M.; Adlassnig, W.; Hofhansl, F.; Bock, B.; Lang, I. Zinc and Copper Uptake in Physcomitrella Patens: Limitations and Effects on Growth and Morphology. Environ. Exp. Bot. 2015, 118, 12–20.
- Krzesłowska, M.; Lenartowska, M.; Samardakiewicz, S.; Bilski, H.; Woźny, A. Lead Deposited in the Cell Wall of Funaria Hygrometrica Protonemata Is Not Stable—A Remobilization Can Occur. Environ. Pollut. 2010, 158, 325–338.
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51.
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2007, 12, 267–277.
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900.
- BUCK, G.W.; BROWN, D.H. The Effect of Desiccation on Cation Location in Lichens. Ann. Bot. 1979, 44, 265–277.
- Itouga, M.; Hayatsu, M.; Sato, M.; Tsuboi, Y.; Kato, Y.; Toyooka, K.; Suzuki, S.; Nakatsuka, S.; Kawakami, S.; Kikuchi, J.; et al. Protonema of the Moss Funaria Hygrometrica Can Function as a Lead (Pb) Adsorbent. PLoS ONE 2017, 12, e0189726.
- Krzesłowska, M.; Lenartowska, M.; Mellerowicz, E.J.; Samardakiewicz, S.; Woźny, A. Pectinous Cell Wall Thickenings Formation-A Response of Moss Protonemata Cells to Lead. Environ. Exp. Bot. 2009, 65, 119–131.
- Kováčik, J.; Babula, P.; Hedbavny, J. Comparison of Vascular and Non-Vascular Aquatic Plant as Indicators of Cadmium Toxicity. Chemosphere 2017, 180, 86–92.
- Kováčik, J.; Dresler, S.; Babula, P. Long-Term Impact of Cadmium in Protonema Cultures of Physcomitrella Patens. Ecotoxicol. Environ. Saf. 2020, 193, 110333.
- Dazy, M.; Masfaraud, J.-F.; Férard, J.-F. Induction of Oxidative Stress Biomarkers Associated with Heavy Metal Stress in Fontinalis Antipyretica Hedw. Chemosphere 2009, 75, 297–302.
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and Structural Biomarkers to Monitor Heavy Metal Pollution of One of the Most Contaminated Freshwater Sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673.
- Maresca, V.; Sorbo, S.; Loppi, S.; Funaro, F.; Del Prete, D.; Basile, A. Biological Effects from Environmental Pollution by Toxic Metals in the “Land of Fires” (Italy) Assessed Using the Biomonitor Species Lunularia Cruciata L. (Dum). Environ. Pollut. 2020, 265, 115000.
- Maresca, V.; Lettieri, G.; Sorbo, S.; Piscopo, M.; Basile, A. Biological Responses to Cadmium Stress in Liverwort Conocephalum Conicum (Marchantiales). Int. J. Mol. Sci. 2020, 21, 6485.
- Maresca, V.; Salbitani, G.; Moccia, F.; Cianciullo, P.; Carraturo, F.; Sorbo, S.; Insolvibile, M.; Carfagna, S.; Panzella, L.; Basile, A. Antioxidant Response to Heavy Metal Pollution of Regi Lagni Freshwater in Conocephalum Conicum L. (Dum.). Ecotoxicol. Environ. Saf. 2022, 234, 113365.
- Basile, A.; Sorbo, S.; Conte, B.; Cardi, M.; Esposito, S. Ultrastructural Changes and Heat Shock Proteins 70 Induced by Atmospheric Pollution Are Similar to the Effects Observed under in Vitro Heavy Metals Stress in Conocephalum Conicum (Marchantiales--Bryophyta). Environ. Pollut. 2013, 182, 209–216.
- Basile, A.; Sorbo, S.; Cardi, M.; Lentini, M.; Castiglia, D.; Cianciullo, P.; Conte, B.; Loppi, S.; Esposito, S. Effects of Heavy Metals on Ultrastructure and Hsp70 Induction in Lemna Minor L. Exposed to Water along the Sarno River, Italy. Ecotoxicol. Environ. Saf. 2015, 114, 93–101.
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The Biological Response Chain to Pollution: A Case Study from the “Italian Triangle of Death” Assessed with the Liverwort Lunularia Cruciata. Environ. Sci. Pollut. Res. 2017, 24, 26185–26193.
- Basile, A.; Sorbo, S.; Lentini, M.; Conte, B.; Esposito, S. Water Pollution Causes Ultrastructural and Functional Damages in Pellia Neesiana (Gottsche) Limpr. J. Trace Elem. Med. Biol. 2017, 43, 80–86.
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Karam, E.A.; Lentini, M.; et al. In-Field and in-Vitro Study of the Moss Leptodictyum Riparium as Bioindicator of Toxic Metal Pollution in the Aquatic Environment: Ultrastructural Damage, Oxidative Stress and HSP70 Induction. PLoS ONE 2018, 13, e0195717.
More
