Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by CHARALAMPIA AMERIKANOU.
Functional dyspepsia is a gastrointestinal disorder characterized by postprandial fullness, early satiation, epigastric pain, and epigastric burning. The pathophysiology of the disease is not fully elucidated and there is no permanent cure, although some therapies (drugs or herbal remedies) try to reduce the symptoms.
- functional dyspepsia
- epigastric pain syndrome
- postprandial distress syndrome
- foods
- dietary patterns
- eating behavior
- nutrition
- diet
1. Introduction
Functional dyspepsia (FD) is considered to be one of the most common disorders in clinical practice [1]. It has a high prevalence that affects 10–30% of adults and 3.5–27% of children worldwide [2]. Despite its high prevalence, there are major uncertainties regarding its definition, pathophysiology, diagnosis, treatment, and prognosis [1].
2. Pathophysiology
Although the etiology of the disorder has not been fully elucidated, the main pathophysiological mechanisms that have been proposed throughout the years include motility alterations and psychosocial factors. The disruption of the microbiota–gut–brain axis, with abnormal central modulation, visceral hypersensitivity, and increased mucosal permeability contribute to the pathophysiology of FD. Increased intestinal permeability, immune activation, and gut dysbiosis caused by stress, which in turn affect the nervous system, suggests the concept of an impaired bidirectional communication of the “brain–gut axis” in FD [3]. In addition, acute enteric infections lead to colonic inflammation, recruitment of eosinophils and mast cells, lymphoid follicles, and duodenal mucosal bacterial loads, which affect the symptomatology of FD patients. Increased levels of inflammatory cytokines in the colonic mucosa are associated with anxiety and depression, which are related to the gut–brain axis [4]. Finally, genetic factors (such as polymorphisms in the genes related to gastrointestinal mobility and immune function), Helicobacter pylori infection, and impaired duodenal mucosal barrier function have been linked with worse FD symptoms [5,6,7][5][6][7] (Figure 1).
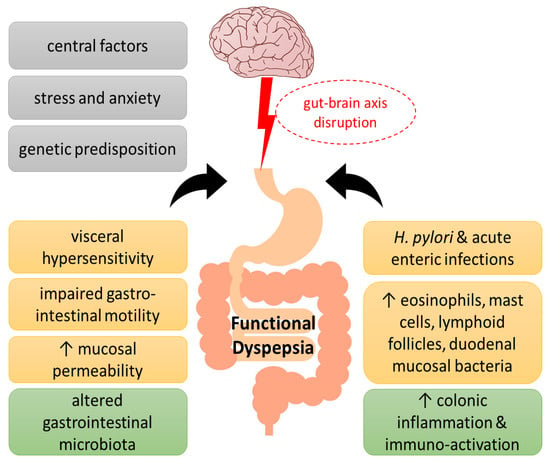
Figure 1. Pathogenesis of functional dyspepsia (FD). A series of pathogenic factors have been proposed for FD, including central nervous system abnormalities and genetic predisposition, as well as psychological factors, which have been suggested to interfere with the gut–brain axis function. Visceral hypersensitivity, impaired gastrointestinal motility, increased epithelial barrier permeability of the duodenal mucosa and infections, such as Helicobacter pylori, have been associated with altered intestinal flora in FD towards immune activation, immune cell infiltration, and low-grade inflammation.
3. Clinical Manifestations
According to the Rome IV criteria for the diagnosis of functional dyspepsia, the main clinical symptoms include bothersome postprandial fullness, early satiation, epigastric pain, and/or epigastric burning along with the absence of any structural disease that may explain the symptoms. Furthermore, the above symptomatology impairs the patient’s quality of life and emotional health, and creates significant financial burden due to increased medical expenses and reduced work productivity. [8]. FD symptoms must be present for a minimum of 3 days a week during the last 3 months, they must be chronic, and start at least 6 months before diagnosis [9]. FD diagnosis includes an evaluation of the clinical history, physical examination, minimal laboratory tests, and a normal upper endoscopy. It is further categorized into epigastric pain syndrome (EPS) and eating-related postprandial distress syndrome (PDS). PDS is defined by bothersome postprandial fullness, that can affect typical activities, and/or bothersome early satiation, that can prevent the completion of a regular-sized meal. EPS is defined by bothersome epigastric pain and/or epigastric burning, both severe enough to disturb usual activities. The Rome IV classification involves not only PDS and EPS, but also their overlap (PDS-EPS overlapped syndrome), which is observed more frequently in hospital than in the general population [8].
4. Medicines
As there is no standard treatment for FD, research on effective therapies is ongoing, but still needs further confirmation. The acid-suppressive therapy with proton pump inhibitors (PPIs) is the most common treatment method [10]. Treatment with the tetracyclic antidepressant mirtazapine improves the quality of life, and buspirone, a serotonin-1A receptor agonist, can alleviate FD symptoms [11]. Prokinetics facilitate the gastric emptying rate [12], while amitriptyline, a neuromodulator, seems to be less effective for the treatment of FD [13]. Finally, the antibiotic rifaximin can change the duodenal microenvironment and reduce FD symptoms [14].
5. Herbal Remedies
Several herbal remedies have been proven effective and safe in FD with comparable outcomes with conventional treatments, and can serve as complementary and alternative medicine, especially when first line therapeutic approaches fail or are inaccessible to patients [9]. Some herbal oils improve PDS and EPS, and improve gastrointestinal symptom rating scale (GSRS) numbers and quality of life scores [15]. Herbal treatments show anti-inflammatory effects and contribute to an improvement in the function of gut microbiota, immune system, central stimuli, and intestinal motility in FD [16]. A systematic review and meta-analysis of 23 randomized controlled trials (RCTs) comparing the effectiveness of herbal treatments versus a placebo or other standard treatments for FD found that the majority of participants (>60%) in the herbal treatment group experienced an improvement in symptomatology and quality of life, compared to participants in the placebo group [17]. Chinese herbal medicines have been considered an effective alternative to prokinetics, according to a meta-analysis of 28 RCTs showing that Chinese herbal remedies were more effective than prokinetics at reducing the overall symptoms [18]. A combination of three herbs (Trachyspermum ammi L., Anethum graveolens L., and Zataria multiflora Boiss) may be important in the treatment of FD, as the essential oils were proven more effective than omeprazole [15]. Similarly, the Japanese Yukgunja-tang, also known as Rikkunshito, is a mixture of eight herbs that is frequently prescribed in FD [19], and it was proven more effective in the total clinical efficacy rate in a meta-analysis of 10 studies with 1246 patients, when combined with Western medicine over the use of Western medicine alone [20]. Additionally, perilla/ginger nutraceuticals have been shown to ameliorate some FD symptoms, such as epigastric pain, heartburn, and gastric reflux, with minor adverse events [21]. Artichoke leaf extract supplementation resulted in a greater amelioration of the multiple correspondence analysis scale compared to a placebo [22], while ginger accelerated gastric emptying [23]. The use of peppermint and caraway oil, a combination with unique properties, showed a statistically significant effect in the global improvement of FD symptoms in a meta-analysis of five RCTs [24]. A unique Greek herbal remedy known as Chios mastic gum has been shown to alleviate the symptoms of FD when taken daily for three weeks over a placebo [25]. The Hong Kong index of dyspepsia was used to assess the efficacy of the mastic treatment.
References
- Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; Accarino, A.; Barbara, G.; Bor, S.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. United Eur. Gastroenterol. J. 2021, 9, 307–331.
- Drago, L.; Meroni, G.; Pistone, D.; Pasquale, L.; Milazzo, G.; Monica, F.; Aragona, S.; Ficano, L.; Vassallo, R. Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies. J. Int. Med. Res. 2021, 49, 0300060520982657.
- Wauters, L.; Talley, N.J.; Walker, M.M.; Tack, J.; Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2019, 69, 591–600.
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35 (Suppl. S1), 5–13.
- Wauters, L.; Li, H.; Talley, N.J. Editorial: Disruption of the Microbiota-Gut-Brain Axis in Functional Dyspepsia and Gastroparesis: Mechanisms and Clinical Implications. Front. Neurosci. 2022, 16, 941810.
- Komori, K.; Ihara, E.; Minoda, Y.; Ogino, H.; Sasaki, T.; Fujiwara, M.; Oda, Y.; Ogawa, Y. The Altered Mucosal Barrier Function in the Duodenum Plays a Role in the Pathogenesis of Functional Dyspepsia. Dig. Dis. Sci. 2019, 64, 3228–3239.
- Wauters, L.; Burns, G.; Ceulemans, M.; Walker, M.M.; Vanuytsel, T.; Keely, S.; Talley, N.J. Duodenal inflammation: An emerging target for functional dyspepsia? Expert Opin. Ther. Targets 2020, 24, 511–523.
- Stanghellini, V.; Chan, F.K.L.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392.
- Duboc, H.; Latrache, S.; Nebunu, N.; Coffin, B. The Role of Diet in Functional Dyspepsia Management. Front. Psychiatry 2020, 11, 23.
- Moayyedi, P.M.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013.
- Tack, J.; Janssen, P.; Masaoka, T.; Farré, R.; Van Oudenhove, L. Efficacy of Buspirone, a Fundus-Relaxing Drug, in Patients With Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2012, 10, 1239–1245.
- Pittayanon, R.; Yuan, Y.; Bollegala, N.P.; Khanna, R.; Lacy, B.E.; Andrews, C.N.; Leontiadis, G.I.; Moayyedi, P. Prokinetics for Functional Dyspepsia. Am. J. Gastroenterol. 2019, 114, 233–243.
- Talley, N.J.; Locke, G.R.; Saito, Y.A.; Almazar, A.E.; Bouras, E.P.; Howden, C.W.; Lacy, B.E.; DiBaise, J.K.; Prather, C.M.; Abraham, B.P.; et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 2015, 149, 340–349.e2.
- Meyrat, P.; Safroneeva, E.; Schoepfer, A.M. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment. Pharmacol.Ther. 2012, 36, 1084–1093.
- Bordbar, G.; Miri, M.B.; Omidi, M.; Shoja, S.; Akhavan, M. Comparison of a Novel Herbal Medicine and Omeprazole in the Treatment of Functional Dyspepsia: A Randomized Double-Blinded Clinical Trial. Gastroenterol. Res. Pract. 2020, 2020, 5152736.
- Kim, Y.S.; Kim, J.-W.; Ha, N.-Y.; Kim, J.; Ryu, H.S. Herbal Therapies in Functional Gastrointestinal Disorders: A Narrative Review and Clinical Implication. Front. Psychiatry 2020, 11, 601.
- Heiran, A.; Bagheri Lankarani, K.; Bradley, R.; Simab, A.; Pasalar, M. Efficacy of herbal treatments for functional dyspepsia: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2021, 36, 686–704.
- Ho, L.; Zhong, C.C.W.; Wong, C.H.L.; Wu, J.C.Y.; Chan, K.K.H.; Wu, I.X.Y.; Leung, T.H.; Chung, V.C.H. Chinese herbal medicine for functional dyspepsia: A network meta-analysis of prokinetic-controlled randomised trials. Chin. Med. 2021, 16, 140.
- Yoon, J.Y.; Ko, S.-J.; Park, J.-W.; Cha, J.M. Complementary and alternative medicine for functional dyspepsia: An Asian perspective. Medicine 2022, 101, e30077.
- Ko, S.; Park, J.; Kim, M.; Kim, J.; Park, J. Effects of the herbal medicine Rikkunshito, for functional dyspepsia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 64–74.
- Di Pierro, F.; Giovannone, M.; Saponara, M.; Ivaldi, L. Effectiveness of a nutraceutical supplement containing highly standardized perilla and ginger extracts in patients with functional dyspepsia. Minerva Gastroenterol. Dietol. 2020, 66, 35–40.
- Giacosa, A.; Guido, D.; Grassi, M.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Perna, S.; Faliva, M.A.; Rondanelli, M. The Effect of Ginger (Zingiber officinalis) and Artichoke (Cynara cardunculus) Extract Supplementation on Functional Dyspepsia: A Randomised, Double-Blind, and Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 915087.
- Hu, M.-L. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J. Gastroenterol. 2011, 17, 105.
- Li, J.; Lv, L.; Zhang, J.; Xu, L.; Zeng, E.; Zhang, Z.; Wang, F.; Tang, X. A Combination of Peppermint Oil and Caraway Oil for the Treatment of Functional Dyspepsia: A Systematic Review and Meta-Analysis. Evid. -Based Complement. Altern. Med. 2019, 2019, 7654947.
- Dabos, K.J.; Sfika, E.; Vlatta, L.J.; Frantzi, D.; Amygdalos, G.I.; Giannikopoulos, G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. J. Ethnopharmacol. 2010, 127, 205–209.
More
