Ganglioside GD3 is a major ganglioside in neuronal progenitor cells. Highly sialylated gangliosides, GM1, GD1a, GD1b, GT1b are the main gangliosides in adult neurons. GD3 is implicated in cell attachment and cell-to-cell interaction during embryogenesis. Anti-ganglioside GD3 monoclonal antibody (clone:R24) coimmunoprecipitates heterotrimeric G protein Goα, GPI-anchored neuronal cell adhesion molecule TAG-1, Src-family kinase Lyn and Csk -binding protein Cbp from rat cerebellar granule cells. Ganglioside GD3 is involved in the migration of granule cells during the early stage of cerebellar development via these GD3-binding proteins.
- gangliosides
- GPI-anchored proteins
- Src-family kinases
- lipid rafts
- heterotrimeric G proteins
- GD3
- cerebellar granule cells
- migration
- phosphacan
- chondroitin sulfate proteoglycans
1. Gangliosides
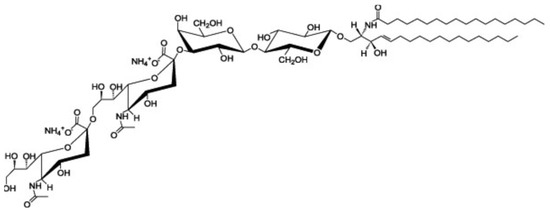
2. Ganglioside GD3-Binding Proteins in Cerebellar Granule Cells
The cerebellar cortex is organized in four layers, including the external granular layer (EGL), molecular layer (ML), Purkinje cell layer (PCL), and internal granular layer (IGL) during development. Granule cells pass through all the cortical layers of the cerebellum [8]. First, granule progenitor cells migrate tangentially within the EGL, where they differentiate into immature granule cells. Stromal-cell derived factor-1 (SDF-1α), a chemokine expressed by meninges (also known as CXCL12), is an attractive guidance cue for tangential migration and a meningeal attractant of granule cells [9]. Immature cerebellar granule cells expressing the CXCR4 receptor migrate under the pial surface and meninges in response to the SDF-1α attractive guidance cue. SDF-1α prevents radial migration by chemoattracting granule cells toward the pia. These immature granule cells pause within the premigratory zone of the EGL before migrating through the ML and PCL to the IGL, and then they change their direction by migrating radially along the processes of Bergmann glial cells through the ML. However, the mechanism by which immature granule cells pause at the EGL/ML interface remains obscure.
2.1. Heterotrimeric G Protein Goα
2.2. Src-Family Kinase Lyn
2.3. GPI-Anchored Neuronal Cell Adhesion Molecule TAG-1
2.4. Csk-Binding Protein Cbp
- Yamakawa, T.; and Nagai, Y. Glycolipids at the cell surface and their biological functions. Trends Biochem. Sci. 1978, 3, 128–131.
- Hakomori, S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem 1981, 50, 733-764, doi:10.1146/annurev.bi.50.070181.003505.
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533-544, doi:10.1016/0092-8674(92)90189-j.
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 2010, 11, 688-699, doi:10.1038/nrm2977.
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Mori, A.; Sugiura, Y.; Furukawa, K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc Natl Acad Sci U S A 2009, 106, 22405-22410, doi:10.1073/pnas.0912336106.
- Furukawa, K.; Ohmi, Y.; Yesmin, F.; Tajima, O.; Kondo, Y.; Zhang, P.; Hashimoto, N.; Ohkawa, Y.; Bhuiyan, R.H. Novel Molecular Mechanisms of Gangliosides in the Nervous System Elucidated by Genetic Engineering. Int J Mol Sci 2020, 21, doi:10.3390/ijms21061906.
- Nara, K.; Watanabe, Y.; Maruyama, K.; Kasahara, K.; Nagai, Y.; Sanai, Y. Expression cloning of a CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1'Cer alpha 2,8-sialyltransferase (GD3 synthase) from human melanoma cells. Proc Natl Acad Sci U S A 1994, 91, 7952-7956, doi:10.1073/pnas.91.17.7952.
- Galas, L.; Bénard, M.; Lebon, A.; Komuro, Y.; Schapman, D.; Vaudry, H.; Vaudry, D.; Komuro, H. Postnatal Migration of Cerebellar Interneurons. Brain Sci 2017, 7, doi:10.3390/brainsci7060062.
- Zhu, Y.; Yu, T.; Zhang, X.C.; Nagasawa, T.; Wu, J.Y.; Rao, Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci 2002, 5, 719-720, doi:10.1038/nn881.
- Yuyama, K.; Sekino-Suzuki, N.; Sanai, Y.; Kasahara, K. Translocation of activated heterotrimeric G protein Galpha(o) to ganglioside-enriched detergent-resistant membrane rafts in developing cerebellum. J Biol Chem 2007, 282, 26392-26400, doi:10.1074/jbc.M705046200.
- Chédotal, A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci 2010, 33, 163-172, doi:10.1016/j.tins.2010.01.004.
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 1998, 95, 9448-9453, doi:10.1073/pnas.95.16.9448.
- Kasahara, K.; Watanabe, Y.; Yamamoto, T.; Sanai, Y. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J Biol Chem 1997, 272, 29947-29953, doi:10.1074/jbc.272.47.29947.
- Kasahara, K.; Watanabe, K.; Takeuchi, K.; Kaneko, H.; Oohira, A.; Yamamoto, T.; Sanai, Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J Biol Chem 2000, 275, 34701-34709, doi:10.1074/jbc.M003163200.
- Kasahara, K.; Watanabe, K.; Kozutsumi, Y.; Oohira, A.; Yamamoto, T.; Sanai, Y. Association of GPI-anchored protein TAG-1 with src-family kinase Lyn in lipid rafts of cerebellar granule cells. Neurochem Res 2002, 27, 823-829, doi:10.1023/a:1020265225916.
- Milev, P.; Maurel, P.; Häring, M.; Margolis, R.K.; Margolis, R.U. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J Biol Chem 1996, 271, 15716-15723, doi:10.1074/jbc.271.26.15716.
- Komatsuya, K.; Iguchi, T.; Fukuyama, M.; Kawashima, I.; Ogura, K.; Kikuchi, N.; Shimoda, Y.; Takeda, Y.; Shimonaka, M.; Yamamoto, N.; et al. Phosphacan acts as a repulsive cue in murine and rat cerebellar granule cells in a TAG-1/GD3 rafts-dependent manner. J Neurochem 2022, 163, 375-390, doi:10.1111/jnc.15709.
- Sekino-Suzuki, N.; Yuyama, K.; Miki, T.; Kaneda, M.; Suzuki, H.; Yamamoto, N.; Yamamoto, T.; Oneyama, C.; Okada, M.; Kasahara, K. Involvement of gangliosides in the process of Cbp/PAG phosphorylation by Lyn in developing cerebellar growth cones. J Neurochem 2013, 124, 514-522, doi:10.1111/jnc.12040.
- Santiago, M.F.; Scemes, E. Neuroblast migration and P2Y(1) receptor mediated calcium signalling depend on 9-O-acetyl GD3 ganglioside. ASN Neuro 2012, 4, 357-369, doi:10.1042/AN20120035.
References
- Yamakawa, T.; and Nagai, Y. Glycolipids at the cell surface and their biological functions. Trends Biochem. Sci. 1978, 3, 128–131.
- Hakomori, S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem 1981, 50, 733-764, doi:10.1146/annurev.bi.50.070181.003505.
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533-544, doi:10.1016/0092-8674(92)90189-j.
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 2010, 11, 688-699, doi:10.1038/nrm2977.
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Mori, A.; Sugiura, Y.; Furukawa, K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc Natl Acad Sci U S A 2009, 106, 22405-22410, doi:10.1073/pnas.0912336106.
- Furukawa, K.; Ohmi, Y.; Yesmin, F.; Tajima, O.; Kondo, Y.; Zhang, P.; Hashimoto, N.; Ohkawa, Y.; Bhuiyan, R.H. Novel Molecular Mechanisms of Gangliosides in the Nervous System Elucidated by Genetic Engineering. Int J Mol Sci 2020, 21, doi:10.3390/ijms21061906.
- Nara, K.; Watanabe, Y.; Maruyama, K.; Kasahara, K.; Nagai, Y.; Sanai, Y. Expression cloning of a CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1'Cer alpha 2,8-sialyltransferase (GD3 synthase) from human melanoma cells. Proc Natl Acad Sci U S A 1994, 91, 7952-7956, doi:10.1073/pnas.91.17.7952.
- Galas, L.; Bénard, M.; Lebon, A.; Komuro, Y.; Schapman, D.; Vaudry, H.; Vaudry, D.; Komuro, H. Postnatal Migration of Cerebellar Interneurons. Brain Sci 2017, 7, doi:10.3390/brainsci7060062.
- Zhu, Y.; Yu, T.; Zhang, X.C.; Nagasawa, T.; Wu, J.Y.; Rao, Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci 2002, 5, 719-720, doi:10.1038/nn881.
- Yuyama, K.; Sekino-Suzuki, N.; Sanai, Y.; Kasahara, K. Translocation of activated heterotrimeric G protein Galpha(o) to ganglioside-enriched detergent-resistant membrane rafts in developing cerebellum. J Biol Chem 2007, 282, 26392-26400, doi:10.1074/jbc.M705046200.
- Chédotal, A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci 2010, 33, 163-172, doi:10.1016/j.tins.2010.01.004.
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 1998, 95, 9448-9453, doi:10.1073/pnas.95.16.9448.
- Kasahara, K.; Watanabe, Y.; Yamamoto, T.; Sanai, Y. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J Biol Chem 1997, 272, 29947-29953, doi:10.1074/jbc.272.47.29947.
- Kasahara, K.; Watanabe, K.; Takeuchi, K.; Kaneko, H.; Oohira, A.; Yamamoto, T.; Sanai, Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J Biol Chem 2000, 275, 34701-34709, doi:10.1074/jbc.M003163200.
- Kasahara, K.; Watanabe, K.; Kozutsumi, Y.; Oohira, A.; Yamamoto, T.; Sanai, Y. Association of GPI-anchored protein TAG-1 with src-family kinase Lyn in lipid rafts of cerebellar granule cells. Neurochem Res 2002, 27, 823-829, doi:10.1023/a:1020265225916.
- Milev, P.; Maurel, P.; Häring, M.; Margolis, R.K.; Margolis, R.U. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J Biol Chem 1996, 271, 15716-15723, doi:10.1074/jbc.271.26.15716.
- Komatsuya, K.; Iguchi, T.; Fukuyama, M.; Kawashima, I.; Ogura, K.; Kikuchi, N.; Shimoda, Y.; Takeda, Y.; Shimonaka, M.; Yamamoto, N.; et al. Phosphacan acts as a repulsive cue in murine and rat cerebellar granule cells in a TAG-1/GD3 rafts-dependent manner. J Neurochem 2022, 163, 375-390, doi:10.1111/jnc.15709.
- Sekino-Suzuki, N.; Yuyama, K.; Miki, T.; Kaneda, M.; Suzuki, H.; Yamamoto, N.; Yamamoto, T.; Oneyama, C.; Okada, M.; Kasahara, K. Involvement of gangliosides in the process of Cbp/PAG phosphorylation by Lyn in developing cerebellar growth cones. J Neurochem 2013, 124, 514-522, doi:10.1111/jnc.12040.
- Santiago, M.F.; Scemes, E. Neuroblast migration and P2Y(1) receptor mediated calcium signalling depend on 9-O-acetyl GD3 ganglioside. ASN Neuro 2012, 4, 357-369, doi:10.1042/AN20120035.

