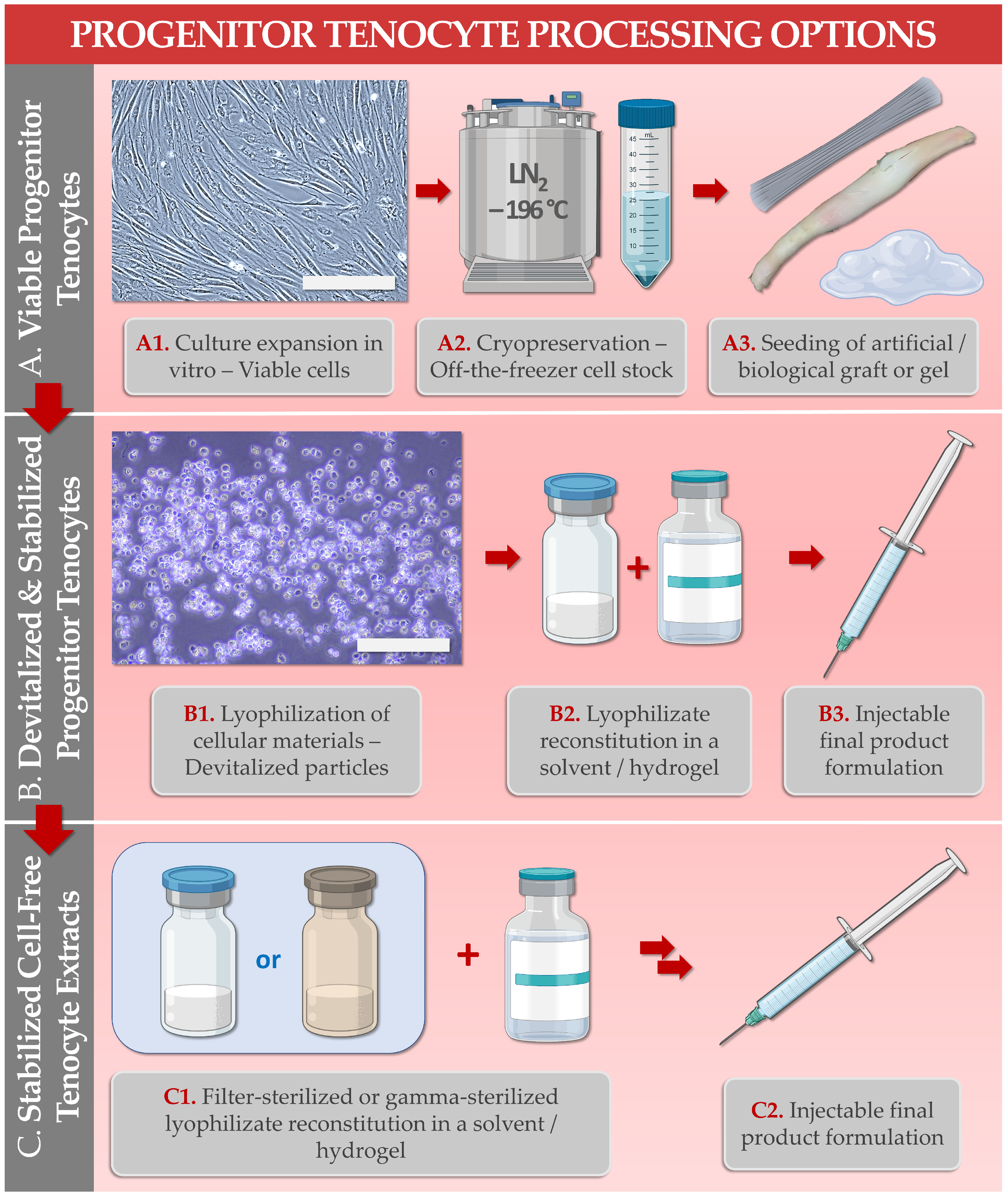
Primary progenitor tenocytes are diploid cells that may be cultured in vitro and therapeutically used for allogeneic musculoskeletal regenerative medicine. Firstly, technical aspects of cell banking, biotechnological manufacturing, and extensive preclinical characterization data have confirmed that FE002-Ten primary progenitor tenocytes may be safely considered for human cytotherapeutic use (e.g., in tissue engineering products, standardized transplants). Parallelly, lyophilized progenitor tenocyte extracts (e.g., stabilized cells or cell-free derivatives) were shown to optimally act as potent hyaluronan-based hydrogel functionalizing agents, useful for stability enhancement against oxidative product degradation. Therefore, primary progenitor tenocytes (e.g., FE002-Ten cell source) may potentially be used in diverse clinical presentations of tendon-related pathologies, ranging from volumetric tissue replacement (i.e., for the promotion of enhanced graft bio-integration) to local management of tissular inflammation and pain (i.e., ancillary action of the cellular extracts for the functional enhancement of injectable hyaluronan-based preparations). Overall, the primary progenitor tenocytes investigated under the Swiss progenitor cell transplantation program were shown to represent highly standardized biotechnological materials with a versatility of potential therapeutic uses after formulation into an array of cytotherapeutic preparations or cell-free devices.
- bioengineering
- cytotherapeutics
- hyaluronic acid
- hyaluronan-based hydrogels
- preclinical safety
- progenitor tenocytes
- regenerative medicine
- stabilization
- tendinopathies
- translational research
1. Introduction
| Study Subject/Domain | Scope of Study Data/Investigated Parameters | References |
|---|---|---|
| 1. FE002-Ten Cell Source Establishment | Establishment of the FE002-Ten cell source in a cryopreserved multi-tiered biobank following a single controlled organ donation. | [21] |
| 2. FE002-Ten Cell Type In Vitro Characterization | Characterization of primary progenitor tenocyte attributes (e.g., cell population homogeneity and purity, genetic and phenotypic stability, proteomic contents, biological functions) 1. | [9][20][21][22][28] |
| 3. FE002-Ten Cell Type Biobanking & Manufacturing | Establishment of optimized and standardized in vitro primary progenitor tenocyte manufacturing workflows for the production of industrial scale cellular material lots. | [22][28] |
| 4. FE002-Ten Cell Type Preclinical Safety Characterization | Characterization of primary progenitor tenocyte safety (i.e., at clinically relevant passage levels 2) in vitro (e.g., genetic stability, tumorigenicity assays) and in vivo (e.g., CAM model, GLP study of cell implantation in rabbit tendons). | [20][22] |
| 5. FE002-Ten Cell Type Derivative Manufacturing, Lyophilization, and Sterilization | Establishment of biological material processing and purification workflows, for cell-derived and cell-free stabilized formulation obtention. Optimization of pharmaceutical processing (e.g., two-step lyophilization) for temperature stabilization of the cellular extracts. Optimization of the sterilization methodologies (e.g., submicron filtration, 60Co gamma irradiation) for conservation of cell-derived extract critical quality attributes and functional properties. | [27][29] |
| 6. FE002-Ten Cells or Derivatives: Study of Combination Product Prototypes | Translational characterization of primary progenitor tenocytes for tissue engineering applications (e.g., using injectable hydrogels, collagen scaffolds, artificial and biological tendon matrices). Translational characterization of hyaluronan hydrogel-based devices incorporating stabilized cellular derivatives. | [9][20][26][27][29][30] |
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, 32–34.
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653.
- Laurent, A.; Rey, M.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.; Hirt-Burri, N.; Applegate, L.A. Retrospectives on three decades of safe clinical experience with allogeneic dermal progenitor fibroblasts: High versatility in topical cytotherapeutic care. Pharmaceutics 2023, 15, 184.
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344.
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal stem cells empowering tendon regenerative therapies. Int. J. Mol. Sci. 2019, 20, 3002.
- Mirghaderi, S.P.; Valizadeh, Z.; Shadman, K.; Lafosse, T.; Oryadi-Zanjani, L.; Yekaninejad, M.S.; Nabian, M.H. Cell therapy efficacy and safety in treating tendon disorders: A systemic review of clinical studies. J. Exp. Orthop. 2022, 9, 85.
- Kaux, J.F.; Samson, A.; Crielaard, J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2016, 5, 264–269.
- Abate, M.; Schiavone, C.; Salini, V. The use of hyaluronic acid after tendon surgery and in tendinopathies. BioMed Res. Int. 2014, 2014, 783632.
- Grognuz, A.; Scaletta, C.; Farron, A.; Pioletti, D.P.; Raffoul, W.; Applegate, L.A. Stability enhancement using hyaluronic acid gels for delivery of human fetal progenitor tenocytes. Cell Med. 2016, 8, 87–97.
- Masiello, F.; Pati, I.; Veropalumbo, E.; Pupella, S.; Cruciani, M.; De Angelis, V. Ultrasound-guided injection of platelet-rich plasma for tendinopathies: A systematic review and meta-analysis. Blood Transfus. 2022; in press.
- Ho, J.O.; Sawadkar, P.; Mudera, V. A review on the use of cell therapy in the treatment of tendon disease and injuries. J. Tissue Eng. 2014, 5, 2041731414549678.
- Chen, X.; Song, X.H.; Yin, Z.; Zou, X.H.; Wang, L.L.; Hu, H.; Cao, T.; Zheng, M.; Ouyang, H.W. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells 2009, 27, 1276–1287.
- Xu, W.; Wang, Y.; Liu, E.; Sun, Y.; Luo, Z.; Xu, Z.; Liu, W.; Zhong, L.; Lv, Y.; Wang, A.; et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng. Part A 2013, 19, 2439–2451.
- Chong, A.K.; Ang, A.D.; Goh, J.C.; Hui, J.H.; Lim, A.Y.; Lee, E.H.; Lim, B.H. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J. Bone Jt. Surg. Am. 2007, 89, 74–81.
- Van den Boom, N.A.C.; Winters, M.; Haisma, H.J.; Moen, M.H. Efficacy of stem cell therapy for tendon disorders: A systematic review. Orthop. J. Sport. Med. 2020, 8, 2325967120915857.
- Andriolo, L.; Altamura, S.A.; Reale, D.; Candrian, C.; Zaffagnini, S.; Filardo, G. Nonsurgical treatments of patellar tendinopathy: Multiple injections of platelet-rich plasma are a suitable option: A systematic review and meta-analysis. Am. J. Sport. Med. 2019, 47, 1001–1018.
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vasquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet-rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84.
- Van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.J.; Caldwell, D.M.; Verhaar, J.A.; de Vos, R.J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sport. Med. 2021, 55, 249–256.
- Freedman, B.R.; Mooney, D.J.; Weber, E. Advances toward transformative therapies for tendon diseases. Sci. Transl. Med. 2022, 14, eabl8814.
- Grognuz, A.; Scaletta, C.; Farron, A.; Raffoul, W.; Applegate, L.A. Human fetal progenitor tenocytes for regenerative medicine. Cell Transpl. 2016, 25, 463–479.
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758.
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380.
- Zhang, K.; Na, T.; Wang, L.; Gao, Q.; Yin, W.; Wang, J.; Yuan, B.Z. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014, 32, 6820–6827.
- Olshansky, S.J.; Hayflick, L. The role of the WI-38 cell strain in saving lives and reducing morbidity. AIMS Public Health 2017, 4, 127–138.
- US Food and Drug Administration. Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals; FDA: Silver Spring, MD, USA, 1993.
- Aeberhard, P.A.; Grognuz, A.; Peneveyre, C.; McCallin, S.; Hirt-Burri, N.; Antons, J.; Pioletti, D.; Raffoul, W.; Applegate, L.A. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif. Organs 2020, 44, E161–E171.
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163.
- Jeannerat, A.; Peneveyre, C.; Armand, F.; Chiappe, D.; Hamelin, R.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A.; et al. Hypoxic incubation conditions for optimized manufacture of tenocyte-based active pharmaceutical ingredients of homologous standardized transplant products in tendon regenerative medicine. Cells 2021, 10, 2872.
- Laurent, A.; Porcello, A.; Fernandez, P.G.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.; et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics 2021, 13, 2196.
- Liu, H.; Chansoria, P.; Delrot, P.; Angelidakis, E.; Rizzo, R.; Rütsche, D.; Applegate, L.A.; Loterie, D.; Zenobi-Wong, M. Filamented light (flight) biofabrication of highly aligned tissue-engineered constructs. Adv. Mater. 2022, 34, 2204301.
