Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Oluwatoyin A Adeleke and Version 2 by Sirius Huang.
Nanofibrous scaffolds are artificial extracellular matrices that mimic the natural environment for tissue formation. This type of scaffold is more advantageous than other available variants because of its large surface-to-volume ratio, which leads to the efficient promotion of cell adhesion, proliferation, and differentiation.
- skin regeneration
- diabetic wound
- nanofibrous scaffolds
- wound healing
- polymeric biomaterials
- tissue engineering
- self-assembly
- wound dressings
- antibiotics
- phytoconstituents
1. Introduction
The incorporation of various bioactive ingredients into polymer-based dressings, especially for diabetics, has shown promising results in wound care and healing. These bioactive agents include, but are not limited to, antibiotics, phytoconstituents, antioxidants, anti-inflammatory, stem cells, or growth factors (GF) [1][35] (Figure 1).
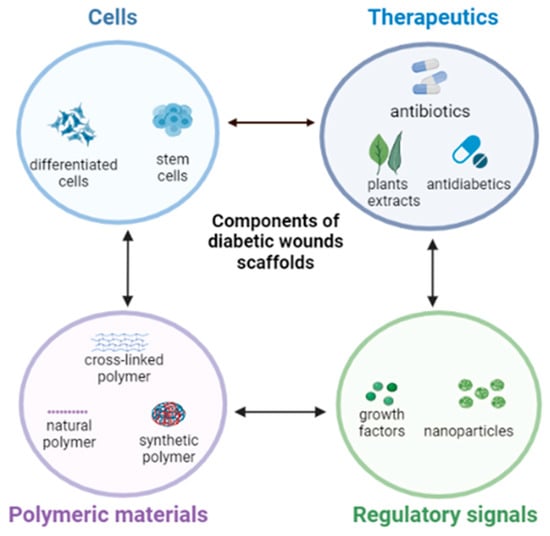
Figure 1.
Typical bioactive components of nanofibrous scaffolds for diabetic wound healing. Create in BioRender.com.
2. Antibiotics
Research has been channeled toward the engineering of antibiotic-loaded nanofibrous scaffolds for wound dressing purposes, such as in diabetic wounds. Such matrices allow localized wound therapy, which turns out to be more selective, effective, and minimizes adverse effects associated with systemic absorption [2][45]. Classes of antibiotics that have been applied for this purpose include aminoglycosides, beta-lactams, quinolones, sulphonamides, tetracyclines, etc. [2][3][45,90].
For instance, Jafari and co-workers [1][35] designed polycaprolactone and gelatin-based bilayered nanofibrous scaffold containing amoxicillin and zinc oxide that prolonged antibacterial effect, quickened wound contraction, elevated collagen deposition and angiogenesis and scar prevention in chronic, full-thickness diabetes wounds. Doxycycline, a broad-spectrum antibiotic, was encapsulated into a polylactide-based nanofiber specifically for the management of chronic wounds. Doxycycline release from the nanofiber was initially rapid and transitioned to a sustained release kinetics at high concentration for two weeks. It also showed a high antibacterial activity, and it inhibited the growth of Escherichia coli and Staphylococcus aureus, which indicates that it is a good candidate for the treatment of infected diabetic lesions [4][91].
Additionally, silver nanoparticles are widely employed in nanofibrous scaffold fabrication due to their antimicrobial property. It has bactericidal effect with decreased ability to cause systemic toxicity. Unlike other antibiotics, it prevents the development of bacterial resistance. Silver nanoparticles can also be combined with antibiotics such as sulphanilamide for synergistic antibacterial (against a wide range of both Gram-positive and Gram-negative bacteria) and wound healing effects [5][6][7][92,93,94]. Other than silver nanoparticles, there are several metal ions (e.g., iron, zinc, titanium, gold, copper, etc.) that possess antibacterial, tissue regeneration, and wound healing properties when fabricated as nanostructures with polymeric biomaterials for diabetic foot ulcers [8][9][10][31,95,96]. Cai and colleagues [11][38] have also reported ferrous oxide loaded onto a gelatin and chitosan nanofiber matrix to form a strong nanofibrous dressing with good antibacterial efficacy for potential diabetic wound dressing application. Another study by Lee et al. [12][32] fabricated coaxial sheath-core nanofibrous poly(lactide-co-glycolide) scaffold sustained the release of vancomycin and gentamicin and sped up the process of healing and repairing early-stage infected diabetic wounds.
3. Herbs and Phytochemicals
Several medicinal plant extracts are being used in the development of scaffolds for diabetic wound dressing, owing to their natural ability to fight off bacteria, act as antioxidant and anti-inflammatory effects with lower toxicity and side effects, low cost, and easy availability [13][97]. Asiaticoside is a phytochemical that possesses numerous therapeutic activities, such as antioxidant, anti-inflammatory, and a potential chronic wound healing ability. Silk-based nanofibrous scaffolds loaded with asiaticoside enhanced the healing of lesions on diabetic induced rat models, and it also exhibited antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus [14][48]. Curcumin is another phytochemical that has strong antioxidant, anti-inflammatory, and anti- infective characteristics [15][98]. Polycaprolactone-based nanofiber loaded with curcumin demonstrated antioxidant and anti-inflammatory effects in diabetic mouse models as compared to nanofibers of polycaprolactone alone [16][33]. In vivo wound closure experiment performed on diabetic rats treated with curcumin-loaded nanofibers showed accelerated healing, and the lesion was completely closed on day fifteen, while the control group showed less than thirty percent closure at the same time point [6][93].
Aloe Vera gel (Aloe barbadensis miller) is another well-known plant chemical known for its therapeutic use in the treatment of burn wounds. It also possesses antidiabetic, anti-inflammatory, and wound-healing abilities by stimulating fibroblast and collagen synthesis to enhance lesion recovery. Aloe Vera gel incorporated into gelatin/polycaprolactone-based nanofiber scaffold was reported to have increased fibroblast proliferation, and it provided antibacterial activity and biodegradability as compared to gelatin/polycaprolactone alone [17][18][19][46,99,100]. Another widely recognized phytochemical known to have potent antioxidant antidiabetic and wound healing properties, which can be beneficial in the treatment of diabetic wounds, is Fenugreek. It was electrospun with silk fibroin and was found to improve collagen deposition at the injured site as well as complete re-epithelialization of the wounded area in a rat model [13][20][41,97]. Selvaraj and colleagues [21][42] further explored Fenugreek extract by incorporating it into a collagen/silk fibroin composite matrix, and they found that this nanofibrous scaffold had antioxidant properties, produced good biocompatibility, and aided fibroblast migration and wound closure through minimal inflammation and early epithelialization. The wound healing efficacy of polyvinyl alcohol/sodium alginate blended nanofibrous scaffolded mats containing Calendula officinalis extract were prepared by electrospinning and tested in male Wistar rat models. Experimental outcomes showed that the scaffolds were biocompatible, and they supported cell attachment and proliferation and injury closure [22][40].
4. Stem Cells
The localized administration of stem cells to open diabetic wounds through nanofibrous scaffold matrices could be a good approach for the enhancement of wound healing due to their ability to secrete immunomodulatory, anti-inflammatory, and angiogenic factors. Although different types of stem cells have been studied, mesenchymal stromal cells (MSC) gained popularity because of their therapeutic use in managing delayed wound healing. MSCs are considered “ideal cell sources for regenerative therapy with no ethical issues” and have shown significant efficacy in the healing of diabetic ulcers. Research has revealed that MSC transplantation can reduce wound dimensions, restore desirable clinical parameters, improve painless walking, and avert amputation related the diabetic foot ulcers [23][101].
A three-dimensional scaffold using polycaprolactone, gelatin, and pluronic-F-127 to administer bone marrow-derived mesenchymal stromal cell (BM-MSC) was developed and was seen to enhance granular tissue formation, angiogenesis, and increased collagen deposition at the wound site in diabetic mouse model [24][49]. Adipose-derived stem cells (ASC) are readily available, possess similar physical and functional characteristics with BM-MSC, and promote diabetic wound healing by increasing tissue regeneration and angiogenesis. It was also reported that ACS promotes cell development by depositing growth factors, such as vascular endothelial growth factor and human growth factor when used topically [25][102]. Fu et al. [26][103] noted the challenges associated with efficiently stabilizing MSC for topical administration due to the high level of proteolysis occurring at the delivery site and therefore engineered a scaffold based on reduced graphene oxide (RGO) nanoparticle combined with an acellular dermal matrix (ADM) that encapsulated MSC. The ADM-RGO scaffold matrix promoted stem cell adhesion and proliferation and was highly stable and mechanically robust. It supported excellent vascularization, collagen deposition, and fast re-epithelization on streptozotocin induced diabetic mice model, presenting a promising therapeutic approach for non-healing diabetic wounds.
In general, there are limited clinical trials reported on the use of MSCs for diabetic wound healing. A few studies on human volunteers, which used BM-MSCs based treatments, were documented, and these continue to serve as progressive evidence demonstrating the efficacy of MSCs in treating diabetic ulcers. Mainly, the injection of autologous transplantation of BM-MSC delivered by intramuscular injection or transplantation [27][28][104,105], directly on wound site [29][30][106,107], by injection into the ischemic limb Procházka et al. [31][108] or via the transfemoral route [32][109] on type 2 diabetic patients with critical limb ischemia and foot ulcers showed significant healing rate with notable improvement in walking (no discomfort), decrease in wound size and healing time, sufficient improvement in leg perfusion and vascularity of skin surrounding wound, increased oxygen pressure, as well as decreased weakness, numbness, and amputation risks.
5. Growth Factors
These are referred to as physiologically active proteins, which are involved in the proliferation, migration differentiation, and metabolism of cells. Together with cytokines, they regulate the healing process that occurs in the body. Nanoparticles loaded with either one or more growth factors showed faster wound healing because growth factors typically promote angiogenesis, inflammatory response, and remodeling. However, because of diabetes, the systemic availability of growth factors decreases [33][110]. Epidermal growth factor (EGF), the most studied growth factor in wound healing, stimulates cell proliferation and differentiation, and a decrease in its concentration has been linked to diabetes mellitus, which is considered one of the factors that contribute to the impaired healing process. Thus, delivering EGF by encapsulating it in suitable polymers, such as collagen hyaluronic acid composite, polyurethane and silk fibroin, has been reported to exhibit anti-inflammatory activity, which further improved wound healing in diabetic rats. Additionally, clinical studies involving the use of EGF incorporated in nano-silver scaffolded dressings displayed a significantly shorter wound repair time and increased granulation tissue in patients with diabetic foot ulcers [34][35][47,111]. Another growth factor that is widely considered for chronic wound treatment is the vascular endothelial growth factor (VEGF) due to its vasculogenic and angiogenic activity. It stimulated cell proliferation, migration of fibroblasts, deposition of collagen, and re-epithelialization when administered through scaffolds in diabetic rats [25][102]. Vijayan and others [36][43] also reported on the construction of nano-encapsulated vascular endothelial growth factor and basic fibroblast growth factors adsorbed onto electrospun collagen/PLGA/chitosan-based scaffolding structures that aided angiogenesis, cell proliferation, collagen deposition, and re-epithelialization at the diabetic wound site. The basic fibroblast growth factor plays a key role in the diabetic wound healing processes, facilitates fibroblast proliferation and neovascularization, and has anti-scaring qualities [36][43].
6. Anti-Inflammatory and Antioxidants
Hyperglycemia promotes the assemblage of reactive oxygen species (ROS) intracellularly, which induces oxidative stress, although oxidative stress is required for wound disinfection and boosts wound healing, and uncontrolled oxidative stress deregulates inflammation and plays a crucial part in the pathogenesis of chronic wounds. Therefore, administering antioxidants help regulates the balance of ROS in the cells [37][112]. Similarly, diabetic patients are more likely to experience microbial-induced inflammation due to skin injuries. Thus, anti-inflammatory agents can be used to prevent and treat that. Glutathione has both antioxidant and anti-inflammatory properties and can be utilized in scaffolds to neutralize excess ROS, as well as to prevent microbial-induced inflammation. Polycaprolactone nanofiber was attached to glutathione soaked in glutaraldehyde solution, which produced a biocompatible and biodegradable characteristic. The outcome shows a promising result that the use of glutathione-polycaprolactone nanofiber could be used for its antioxidant, anti-inflammatory, and possible antibacterial effect due to the presence of glutaraldehyde in the diabetic wound nanofiber-based therapy [38][39].
7. Antidiabetic Agents
Some hypoglycemic agents have been shown to reduce inflammation, a quality that can significantly speed up the healing process of diabetic ulcers and improved therapeutic outcomes. Some examples of antidiabetic agents identified in the literature to have demonstrated moderate to high-level anti-inflammatory activity include sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and metformin, which is a biguanide [39][40][34,113]. In a study conducted by Cam and coworkers [40][113], a combination of oral hypoglycemic drugs, namely, pioglitazone, metformin, and glibenclamide, were embedded within a chitosan/gelatin/polycaprolactone and polyvinyl pyrrolidone composite nanofibrous scaffolds and assessed for their diabetic wound healing effect. This combined therapeutic system quickened diabetic wound healing in rats, improved dermis and epidermis regeneration, and had less inflammatory cell infiltration and oedema. This same group of researchers also reported improvement in in vivo re-epithelialization and formation of granulation tissue in a diabetic wound site by applying a metformin and glibenclamide-loaded gelatin/bacterial cellulose nanofibrous template [39][34].
A collagen/PLGA nanofibrous scaffold membrane was fabricated for sustained release of metformin for wounds associated with diabetes in rat models and the membranes were found to elevate collagen content and effectively promoted wound closure [41][114]. Another study developed a poly (lactic-co-glycolic acid)/gelatin (PLGA/Gel) nanofibrous scaffold mat for the extended release of liraglutide, an antidiabetic agent known to promote angiogenic activities of endothelial cells. Results of the investigation showed a remarkable decrease in the duration of wound closure, increased blood vessel density, and collagen deposition, all facilitating wound repair [42][36]. Besides, a nano-configured lipid carrying pioglitazone (an antidiabetic agent) was embedded into a collagen/chitosan composite scaffold template and examined for diabetic wound healing purposes. The scaffolds were non-toxic and in vitro testing in a streptozotocin-induced diabetic wound model enhanced cell growth, an indication of healing, compared to the control [43][44].
