Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
Eels use the swimbladder for buoyancy control. The ductus pneumaticus connecting the esophagus with the swimbladder is closed soon after initial opening of the swimbladder in the glass eel stage, so that eels are functionally physoclist. Subsequent filling of the swimbladder is achieved by activity of gas gland cells in the swimbladder epithelium and countercurrent concentration in the rete mirabile.
- gas gland
- countercurrent exchange
- metabolism
- Root effect
- pentose phosphate shunt
1. Introduction
Eels are usually considered catadromous fish spawning in the marine habitat, and spending most of their life cycle in freshwater systems. It has been shown, however, that some eels may skip the freshwater phase completely and stay in coastal water, or move between brackish water and freshwater (semi-catadromous behavior) [1]. The spawning area of the European eel Anguilla anguilla (L. 1758) is the Sargasso Sea. In a recent tracking experiment, it was shown for the first time that adult eels released near the Azores swim to the Sargasso Sea [2]. Tagged eels released near the European coast so far could not be tracked all the way down to the Sargasso Sea [3][4][5][6][3,4,5,6]. Fertilized eggs develop into a larvae named Leptocephalus [7], and later it was realized that the Leptocephali are the larvae of the European eel [8]. The Leptocephali drift with the Gulf stream [9][10][9,10] and reach the European or North African continental slope after a journey of about 7 months to 2 years [10]. Before entering the European freshwater system, Leptocephali metamorphose into glass eels. The translucent Leptocephali do not have a swimbladder and appear to be positively buoyant with overall densities of 1.028–1.043 g·mL−1 [11]. This low density value appears to be due to a high concentration of glycosaminoglycans in the translucent extracellular matrix. The swimbladder develops and is first inflated in glass eels. The glass eels then develop into so-called yellow eels, which typically spend 5–25 years in the European freshwater system [9][12][9,12]. In yellow eels, the ductus pneumaticus, i.e. the connection between esophagus and swimbladder is functionally closed, eels cannot take an air breath at the water surface. Filling of the swimbladder is achieved by diffusion of gas from the blood and from gas gland cells in the swimbladder epithelium. The necessary gas partial pressure gradients are generated by activity of swimbladder gas gland cells and countercurrent multiplication in a rete mirabile.
2. Opening of the Swimbladder in Glass Eel
The swimbladder develops as a dorsal outgrowth of the esophagus in early metamorphic stages to the glass eel, Leptocephali do not have a swimbladder [13][20]. In many glass eels caught on arrival along the European Coast, the swimbladder does not yet contain any gas, but is filled with surfactant, which is required to reduce surface tension [13][20]. Its presence has also been shown in yellow eel swimbladder, and it is produced and secreted by gas gland cells [14][21]. In early glass eels, the epithelial gas gland cells do not show the extensive basolateral labyrinth, characteristic for gas gland cells of yellow and silver eels (Figure 1). Moreover, the intimate connection between gas gland cells and blood capillaries is not yet visible [13][20]. Based on the histological appearance of the gas gland cells it therefore was concluded that the initial filling of the swimbladder with gas is not achieved by secretory activity of these cells, but by gulping air or by taking up small gas bubbles from the water [13][20]. Within 3 to 4 months of development after the initial inflation, the connective tissue of the swimbladder and the gas gland cells differentiate to the adult state present in yellow eels with an extensive basolateral labyrinth, which is in close contact with swimbladder capillaries [13][20]. The ductus pneumaticus, i.e. the connection between esophagus and the swimbladder, is functionally closed so that eels cannot gulp air at the water surface. The ductus pneumaticus is used as the resorbing section of the swimbladder with separate blood supply bypassing the rete mirabile. The resorbing section of the swimbladder is separated from the secretory section via a sphincter muscle, located between the two retia mirabilia [15][22].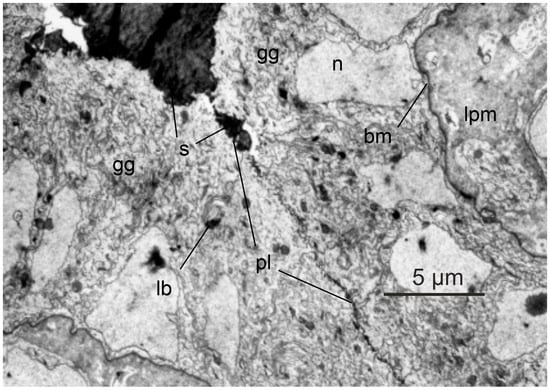
Figure 1. Histology of glass eel swimbladder. In Leptocephali developing into glass eels, the swimbladder initially is filled with surfactant; opposing gas gland cells appear to be separated by a thin layer of surfactant. Gas gland cells do not yet express an extensive basolateral labyrinth and the connection between gas gland cells and blood capillaries is not as tight as in yellow eels. gg, gas gland cell; s, surfactant; n, nucleus; pl, plasma membrane; lb, lamellar body; bm, basal membrane; lpm, lamina propria mucosae. With permission modified after [13][20].
3. Swimbladder Function in Yellow Eels
In the European freshwater system consisting of rivers and lakes, eels experience a limited depth range. Hydrostatic pressure increases by one atmosphere for every ten meters of water depth, so the range of hydrostatic pressures experienced in freshwater is limited. Nevertheless, the swimbladder wall is flexible, and therefore, according to Boyle’s law, in a fish descending from the water surface to a water depth of 10 m, it results in a 50% decrease in swimbladder volume due to the doubling of the hydrostatic pressure. The status of neutral buoyancy, a status in which a fish can stay at a certain water depth without any swimming movement, however, requires a constant swimbladder volume, exactly compensating for the compared to water density higher density of other fish tissues [16][17][23,24]. To avoid compression of the swimbladder when descending into deeper water therefore requires the secretion of gas in order to keep the swimbladder volume constant. Ascending closer to the water surface, in turn, requires the removal of gas to avoid an increase in swimbladder volume resulting in a status of positive buoyancy. Although eels are anatomically physostome, the ductus pneumaticus is functionally closed soon after the original opening of the swimbladder [9][15][9,22]. The yellow eel therefore cannot gulp air at the surface and is functionally a physoclist fish. The thin-walled and almost translucent ductus pneumaticus is used as a resorbing part of the swimbladder. It is highly vascularized, draining into the main venous system. A sphincter muscle located between the two retia mirabilia allows for the transfer of gas from the secretory section to the resorbing part. Along partial pressure gradients, gases then diffuse from this resorbing section to the blood and the venous circulatory system. The wall of the secretory swimbladder has a silvery appearance due to guanine incrustation in connective tissues, and the cell membranes are characterized by a high cholesterol content, resulting in low gas permeability of the swimbladder wall [18][42]. In the eel the whole swimblader epithelium consists of gas gland cells. Arterial blood supply to the secretory swimbladder passes the rete mirabile or red body, a remarkable countercurrent system. In the rete mirabile, the swimbladder artery gives rise to several tens of thousands of capillaries, running in parallel for a distance of several millimeters [19][25]. The capillaries reunify, forming two or three larger arterial vessels supplying the gas gland cells. Venous return to the rete again forms several tens of thousands of venous capillaries running in parallel and surrounding the arterial capillaries in the rete. The diffusion distance between arterial and venous capillaries is in the range of only one to two micrometer [19][25]. This arrangement of blood vessels allows taking blood samples in front of the countercurrent system, i.e., at the heart pole, but also between the rete and the gas gland cells, i.e., at the swimbladder pole. Thus, the concentrating function of the rete mirabile, and the function of gas gland cells can be analyzed separately. This explains why the eel became a model species for the analysis of swimbladder function in physoclist fish [20][26]. In many other species, this separation of rete mirabile and gas gland cell function is not possible, because the two structures are closely connected and rete capillaries do not give raise to larger blood vessels at the swimbladder pole. Blood samples collected at the swimbladder pole therefore may have been influenced by back-diffusion in the rete mirabile, but also by the secretory activity of the gas gland cells [21][22][27,28]. Several in situ studies and also experiments with primary cultured gas gland cells revealed that gas gland cells are specialized for the production of acidic metabolites in the anaerobic metabolism. Oxidation of metabolites in the aerobic metabolism is of minor importance, although gas gland cells are chronically exposed to high oxygen partial pressures. A large fraction of glucose taken up from the blood is converted into lactic acid [23][24][25][26][29,30,31,32]. Some of the glucose taken up from the blood, however, is also shifted to the pentose phosphate shunt, where the activity of 6-phosphogluconate dehydrogenase results in the production of CO2. In fact, CO2 production in the pentose phosphate shunt by far exceeds the amount of CO2 produced in the aerobic metabolism [25][27][28][31,33,34] (Figure 2). The activity of the pentose phosphate shunt also results in the generation of NADPH, an important reduction equivalent involved in the degradation of reactive oxygen species.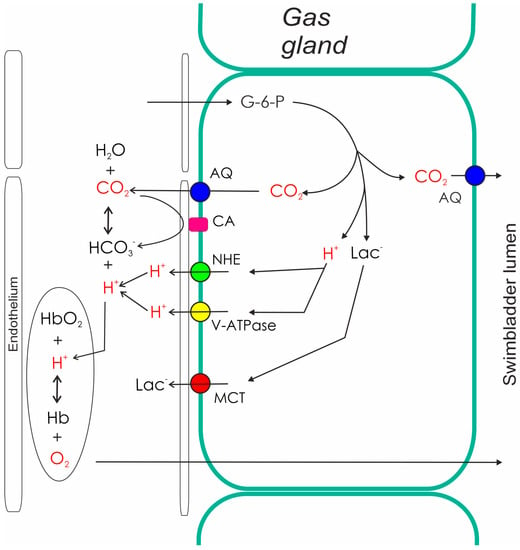
Figure 2. Metabolism and secretory activity of gas gland cells. The metabolism of gas gland cells of the European eel depends on blood glucose. Glucose is converted to lactic acid (lactate anion and a proton), and used for the production of CO2. CO2 production in the pentose phosphate shunt by far exceeds the amount of CO2 produced in the aerobic metabolism. Protons, CO2, and lactate are released into the blood, where the acidification switches on the Root effect. The resulting increase in PO2 provides the partial pressure gradient for the diffusion of oxygen into the swimbladder. The production of CO2 in gas gland cells also generates a partial pressure gradient towards the swimbladder lumen, allowing for the secretion of CO2 into the swimbladder. AQ, aquaporin; CA, carbonic anhydrase; G-6-P, glucose-6-phosphate; Hb, hemoglobin; Lac, lactate; NHE, sodium proton exchanger; MCT, monocarboxylate carrier.
