Consumption of legumes has been shown to enhance health and lower the risk of cardiovascular disease and specific types of cancer. ACE inhibitors, antioxidants, and synthetic anti-inflammatories are widely used today. In vitro and in vivo research has shown the bioactive peptides generated from legume protein hydrolysates, such as antioxidant, anti-hypertensive, anticancer, anti-proliferative, anti-inflammatory, etc., in the context of different disease mitigation. Therefore, researchers describe the recent advances in in vitro and in vivo studies of antioxidant, anti-hypertensive and anti-inflammatory peptides isolated from legume-derived protein hydrolysates. The results indicated that antioxidant legumes peptides are characterized by shortchain sequence amino acids and possess anti-hypertensive properties by reducing systolic blood pressure (SBP) in spontaneously hypertensive rats (SHR).
Consumption of legumes has been shown to enhance health and lower the risk of cardiovascular disease and specific types of cancer. ACE inhibitors, antioxidants, and synthetic anti-inflammatories are widely used today. Recently, in vitro and in vivo research has shown the bioactive peptides generated from legume protein hydrolysates, such as antioxidant, anti-hypertensive, anticancer, anti-proliferative, anti-inflammatory, etc., in the context of different disease mitigation. Therefore, this review aims to describe the recent advances in in vitro and in vivo studies of antioxidant, anti-hypertensive and anti-inflammatory peptides isolated from legume-derived protein hydrolysates. The results indicated that antioxidant legumes peptides are characterized by shortchain sequence amino acids and possess anti-hypertensive properties by reducing systolic blood pressure (SBP) in spontaneously hypertensive rats (SHR).
- legumes
- isolate
- hydrolysate
- bioactive peptides
1. Introduction
2. Legume Protein Isolate and Hydrolysate
The extraction of protein isolates and other components from legume seeds is currently performed using advanced legume extraction and fractionation technologies. Protein isolation can be done in two ways: dry processing for legume seeds, which preserves protein functionality, and wet processing for flours, which yields better protein purity [30][34]. Previous studies have reported on legume protein isolation using the acid-base extraction method, such as chickpea [31][35], black gram [32][36], pea [33][37], pinto beans [34][38], string beans [35][39], and lupin [36][40]. The legume protein isolation begins with extracting the protein in an alkaline solution, followed by isoelectric precipitation (IP), and finally, drying to get the final isolated protein [37][38][41,42]. Los et al. [39][43] extracted carioca bean protein before hydrolysis with NaOH (pH 8.0) and then precipitated it with HCl (pH 4.5). Legume protein hydrolysate can be produced using numerous methods, such as chemical hydrolysis, enzymatic hydrolysis, or microbial fermentation [34][40][38,44]. Enzymatic hydrolysis converts the protein molecules into peptides quickly for various sizes and free amino acids by breaking down certain peptide bonds of the parent protein using proteases with a positive impact on human health [41][45]. In this context, through the extraction of protein isolate, enzymatic hydrolysis, chemical and fermentation synthesis, separation, and purification (ultrafiltration membrane, gel filtration, reverse phase high performance liquid chromatography, and ion exchange chromatography), various legume sources can be converted into antioxidants, ACE-inhibitory, and anti-inflammatory peptides [2][23][42][2,23,31]. These peptides are regarded as a good source and an alternative food for use as antioxidants, antihypertensive, and anti-inflammatory peptides [43][44][45][46,47,48]. Moreover, antioxidant and ACE-inhibitory properties of legume protein hydrolysates are strongly influenced by hydrolysis, which changes the structural and composition characteristics such as molecular weight (MW) [11][46][47][11,49,50]. Putra et al. [13] mentioned that peptide fractions with MW below 1 kDa demonstrated the highest ACE-inhibitory activity among the other fractions derived from pigeon pea hydrolysates because of their capacity to bind Zn2+ and form Peptide- Zn2+ complex, which prevents ACE from using the Zn2+ ions that are already present as a cofactor. According to Chen et al. [48][51], antioxidant peptides were separated from the black bean soybean protein hydrolysate using ultrafiltration, gel filtration (GF), and reverse phase high performance liquid chromatography (RP-HPLC). The amino acid sequence of the sub-fraction (F2-c) peptide (455 Da) was identified as Leu-Val-Pro-Leu-Lys and Ile-Val-Pro-Leu-Lys and has the highest antioxidant of DPPH and HRSA (IC50: 0.12 µM and 0.037 µM).3. In Vitro and In Vivo Studies on the Antioxidant, ACE-Inhibitory, and Anti-Inflammatory Peptides from Legume Protein Hydrolysate
3.1. In-Vitro Study of Antioxidant, ACE-Inhibitory, and Anti-Inflammatory Peptides
3.1.1. Antioxidant Peptide
Reactive oxygen species “(ROS)” are highly m molecules produced by all aerobic cells that can be free radicals, such as hydroxyl radical(•OH), and superoxide radical(•O2) or non-radicals, such as singlet oxygen(1O2) and hydrogen peroxide(H2O2) [49][52]. Free radicals are unavoidable metabolic by-products that are the result of oxidative stress which can damage proteins, cell membranes, phospholipids, and DNA, resulting in severe human diseases such as diabetes, coronary heart disease, hypertension, stroke, cancer, arteriosclerosis, and Alzheimer’s [50][51][53,54]. Therefore, antioxidants play a crucial role in preventing or delaying the autoxidation of food components and the human body by inhibiting oxidation reactions and producing free radicals [52][55]. Antioxidant peptides released during enzymatic hydrolysis act as free radical scavengers, metal inactivators, oxygen inhibitors, or peroxide to protect the body and food system from reactive oxygen species [53][56]. Antioxidants are classified into synthetic and natural categories [54][57]. Although synthetic antioxidants such as “butylated hydroxytoluene (BHT), propyl gallate (PG), butylated hydroxyanisole (BHA), and tert-butyl hydroquinone (TBHQ)” are efficient and relatively cheap, they have displayed some toxic and hazardous properties [55][58]. According to Lourenço et al. [56][59], several studies have been published showing a correlation between the long-term consumption of synthetic antioxidants and specific health problems such as digestive system disorders, skin allergies, and occasionally leading to increased risk of cancer. Additionally, in animal studies, BHT and BHA have already been shown to be responsible for adverse effects on carcinogenesis and the liver. Large dosages of synthetic antioxidants may cause premature senescence and damage the DNA. For this reason, studies have focused on searching for and developing antioxidant peptides from natural sources such as plants and animals [57][60]. These natural antioxidant peptides can be easily absorbed more readily and without adverse side effects compared to synthetic antioxidants. Furthermore, the primary mechanism of these antioxidant peptides is through the ability to inactivate intracellular reactive oxygen species (ROS), scavenge free radicals, reduce lipid peroxidation, and chelate transition metals, among other things, which have all been linked to their antioxidant activities [58][61]. Nevertheless, compared to synthetic antioxidants, some natural antioxidants have lower antioxidant activity, indicating that they need to be used in higher dosages and may result in unsafe dosages. Despite this, if they are taken within the limits of the law, natural antioxidants are a helpful alternative to synthetic ones [56][59]. Food-derived antioxidant peptides are considered natural antioxidant resources which possess acceptable nutritive value, minimal adverse side effects (safe), high efficiency, low molecular weight, high activity, low cost, and are easily absorbable to replace synthetic antioxidants for use in food [49][59][52,62]. Many studies have shown that legumes, such as chickpeas, pigeon peas, beans, soybeans, and lentils, can produce antioxidative peptides in vitro and positively impact human health when used as alternative antioxidants [3]. The antioxidant activity of peptides depends on molecular weight, amino acid composition and sequence, size, hydrophobicity, enzyme specificity, and degree of hydrolysis [20][43][20,46]. Figure 1 shows the main steps for preparing and identifying antioxidant peptides from natural protein sources. In this context, if an antioxidant compound prevents the generation of free alkyl radicals or disrupts the free radical chain’s propagation, the lipid oxidation’s chemical reaction rate is delayed or slowed. The use of singlet oxygen inhibitors, metal-chelating agents, and peroxide stabilizers caused this delay by donating hydrogen from antioxidants and metal-chelating agents [60][63]. Recent interest in antioxidant peptides derived from food proteins has grown owing to their prominent role in the prevention mechanisms of oxidative stresses linked to various diseases [61][64]. Consequently, different methods of in vitro antioxidant systems have been used to assess the antioxidant capacity of legume protein hydrolysates and peptides such as (DPPH) 2,2-diphenyl-1-picrylhydrazyl, (HRSA) hydroxyl radical scavenging activity, (ABTS) 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid radical scavenging, and (ORAC) oxygen radical absorbance capacity, etc. [62][63][65,66]. Lentil protein hydrolysate with an amino acid sequence Ala-Leu-Gly-Pro-Val-Met (587.31 Da) exhibited the highest DPPH (63%) and β-carotene-linoleate model system (73%), respectively [62][65]. Wali et al. [64][67] also mentioned that the identified antioxidant peptide of chickpea protein hydrolysate (NF2-4-1): Leu-Thr-Glu-IIe-IIe-Pro (685.41 Da) showed the highest DPPH and OH scavenging activities, IC50: 0.24 mg/mL and 0.57 mg/mL, respectively. Peptides with low molecular weight have strong antioxidant activities than higher molecular weight peptides because they have a better chance to cross the intestinal barrier to exert antioxidant effects and favor increased interactions with the free radical [65][68].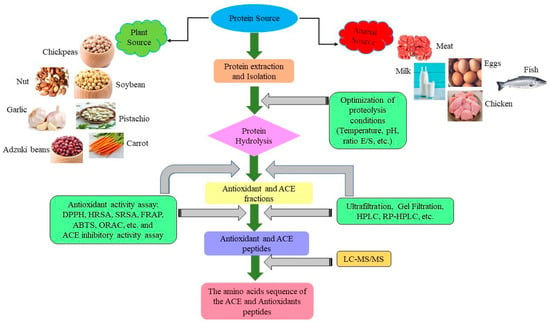
3.1.2. ACE-Inhibitory Peptide
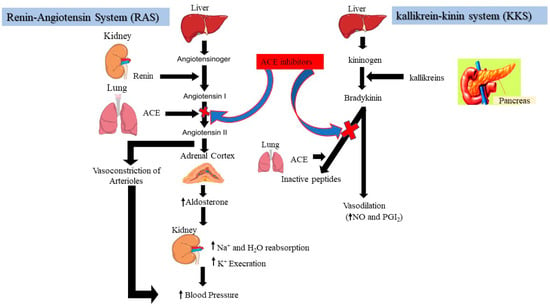
Hypertension, a global issue affecting one-fourth of the world’s adults, is the leading cause of heart disease [68][76]. The two of the most powerful systems for maintaining blood pressure regulation in humans via the angiotensin-I converting enzyme (ACE) are the renin-angiotensin system (RAS) and the kallikrein-kinin system (KKS), as shown in Figure 2. Angiotensinogen is a substrate for renin to produce angiotensin (I), which is then converted into potent vasoconstrictor angiotensin (II) by ACE, causing raised blood pressure [28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. In this context, angiotensin (II) raises oxidative stress when blood pressure is high because it interferes with several of the cell’s functions by increasing the creation of intracellular reactive oxygen species [78]. In contrast, ACE inhibition blocks the first step in the renin-angiotensin system (reduction of angiotensin II), resulting in the treatment of hypertension and enhancing the antioxidative defense system [3]. Inhibitors bind strongly to the ACE active site, contending for occupancy with angiotensin (I); as a result, ACE is unable to convert angiotensin (I) to angiotensin II [79]. Many anti-ACE drugs, such as ramparil, lisinopril, zestril, enalapril, captopril, etc., are commonly used as antihypertensive treatments; with adverse side effects, including taste disturbances, cough, angioedema, and skin rashes [80]. Consequently, natural compounds, especially from plant sources with ACE-inhibitory potential, seem to be of ongoing interest as alternatives to synthetic drugs [81]. Studies have shown that legumes contain beneficial nutrients, bioactive peptides, and high-quality proteins, which have been demonstrated as potent in vitro ACE inhibitors [82].