You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Rui Zhang.
Polychlorinated diphenyl ethers (PCDEs) are a class of synthetic halogenated aromatic compounds, which have gradually attracted widespread attention due to potential environmental risks to humans and ecosystems.
- PCDEs
- persistent substances
- bioaccumulation
1. Introduction
Polychlorinated diphenyl ethers (PCDEs) are a class of synthetic halogenated aromatic compounds comprising 209 possible congeners, which are structurally similar to polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-furans (PCDFs) [1]. However, PCDEs are typically more polar than PCBs due to the presence of the oxygen atom and the resultant asymmetry over the horizontal axis [2,3][2][3]. The theoretical 209 congeners can be divided into ten congener groups from mono- to deca-CDE and numbered (Table S1) according to the International Union of Pure and Applied Chemistry (IUPAC) system established for PCBs [3]. The structural formula of PCDEs is shown in Figure 1 and their molecular formula is C12H10–nClnO (n = 1–10).
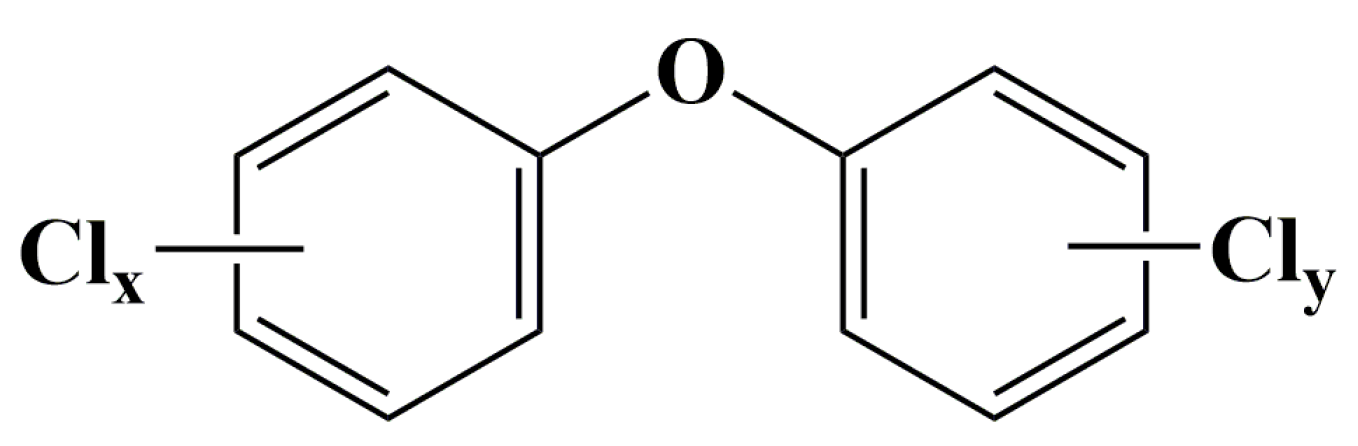
Figure 1.
General chemical structure of polychlorinated diphenyl ethers (x + y ≤ 10).
PCDEs were widely used as flame retardants, hydraulic fluids, electric insulators, lubricants and plasticizers in the 20th century [4,5][4][5]. Currently, congener CDE 13 is used directly as the intermediate in the synthesis of the fungicide difenoconazole [6]. In addition, PCDEs are by-products produced during the synthesis of commercial chlorophenols as important intermediates in the chemical industry [7,8][7][8]. PCDEs can also be generated in the incineration of municipal waste [9,10][9][10]. Therefore, PCDEs have inevitably leaked into the environment and have been detected in water, sediment, soil, atmosphere and various biological samples at total concentrations of 0.351–1800 ng/L, 0–3,980,000 ng/g dry weight (dw), <38–6800 ng/g dw, 8.75 × 10−3–1.15 × 1032 pg/m3 and 0–50,924 ng/g lipid weight (lw), respectively.
Since the negative log-transformed values of 298 K supercooled liquid vapor pressure (PL) of most PCDEs range from approximately 2 to 5, PCDEs may thus transport over long distances with the atmosphere [41][11]. For example, although there were no sources of pollution in the remote Arctic region, PCDEs were also detected in Arctic cod (Arctogadus glacialis) at concentrations of 2–21 ng/g lw [32,42][12][13]. Owing to the strong acid and alkali resistance and antioxidant capacity, PCDEs are persistent in various environmental matrices [1,17,18][1][14][15]. Moreover, the biological half-lives of tetra- to hepta-CDEs generally exceed 100 days in rainbow trout (Salmo gairdneri), which are almost equivalent to those of PCBs [43][16]. In addition, high lipophilicity renders PCDEs susceptible to accumulate in organisms and biomagnify through trophic transfer [44][17]. Toxicokinetic experiments showed that the absorption rate of PCDEs in fish were high at 2.4–48.9 μg/day, and the bioconcentration factor (BCF) could reach 1001–32,000 [44,45,46,47][17][18][19][20]. The bioaccumulation capacities of PCDEs are even higher than those of polychlorinated dibenzo-p-dioxins (PCDDs) and PCDFs in oligochaete worm (Lumbriculus variegatus) [48][21]. In some aquatic food chains, such as oligochaete worm (Lumbriculus variegatus) to white sucker (Catostomus commersoni), the biomagnification factors (BMFs) of PCDEs are 13.7 to 34.6 on a lipid-normalized basis, comparable to those of PCBs [11][22]. In addition, PCDEs have been detected in daily food and health products of humans [9,40][9][23].
Toxicology studies have shown that the toxic effects of PCDEs in organisms are similar to dioxins. During the early life stages of fish, PCDEs may cause embryonic vascular hemorrhage, growth inhibition, deformity and death [49,50][24][25]. PCDEs can also cause oxidative stress in the liver of mice (Mus musculus) and disturb the balance of trace elements [51][26]. Exposure of mice (Mus musculus) to PCDEs during pregnancy resulted in reproductive developmental toxicity, such as reduced survival of fetuses and pups, and disturbed thyroid hormone secretion in maternal and fetal mice [52,53][27][28]. In addition, there is evidence that PCDEs may induce immunotoxicity through the mediation of the aryl hydrocarbon receptor (AHR) [54,55,56][29][30][31]. However, toxicological data of PCDEs are still limited probably due to the paucity of commercially available standards of PCDE congeners. It further leads to insufficient attention to the health and ecological risks brought by PCDEs, along with slowly increasing research on their toxic mechanisms, environmental exposure levels and environmental behavior. In this context, a systematic literature search was performed with a query based on the keywords of “Polychlorinated diphenyl ethers”, “PCDEs” and “polyhalogenated diphenyl ether”. PubMed, Web of Science and Google Scholar were used as search engines/databases. Publishing year was not restricted to retrieve as much of the available literature as possible. The relevant publications on PCDEs, focusing on the sources, environmental level, environmental behavior and fate, synthesis and analysis methods and toxicological research (Figure 2) were screened based on the examination of title, abstract and full text. The results and data were manually extracted and cross-checked by two authors. Additional relevant studies were identified from the reference lists of already identified publications. Finally, a total of 98 relevant publications were obtained from the literature search. Compared with reviews on PCDEs published previously [3[3][7],7], some new information and findings were summarized in this revisewarch: new sources including solid waste incineration [22][32], intermediates [6] and impurities in drugs, daily necessities and pesticides [57][33]; current environmental exposure levels [12,18,21][15][34][35]; main metabolism pathways in different aquatic organisms [58][36]; acute toxicity data for more species and relationships between it and structural parameters [59][37]; and relationships between bioaccumulation potentials and the number/location of substituting Cl atoms of PCDE congeners [58][36]. Furthermore, current research deficiencies were further proposed, and future research perspectives were explored to facilitate the environmental chemistry and toxicology research on PCDEs in the future.

Figure 2.
A schematic representation of the existing knowledge of PCDEs in terms of environmental toxicology and chemistry.
2. Physicochemical Properties
The physicochemical properties related to the environment behavior and fate of pollutants have been determined for 106 PCDE congeners by direct chromatographic methods (Table S2) [41,68][11][38]. However, given the time- and cost-consuming characteristics to evaluate the physicochemical properties experimentally as well as unavailability of standards for the remaining 103 PCDE congeners, various quantitative structure–property relationship (QSPR) methods have been developed and applied to predict the physicochemical properties based on diverse molecular structural descriptors and regression models. For example, seventeen theoretical molecular structural descriptors and partial least squares (PLS) regression were used to predict the PL and n-octanol/water partition coefficient (KOW) of 209 PCDE congeners [69][39]. Linear relationships were established between gas-chromatographic relative retention time (RRT), KOW, PL and aqueous solubility (SW,L) of PCDEs and some structural descriptors derived from molecular surface electrostatic potentials by a multiple linear regression (MLR) method and used to predict the physicochemical properties of PCDE congeners not determined experimentally [70][40]. QSPR models were developed by molecular electronegativity distance vector (MEDV-4) and MLR methods to estimate the PL, KOW and SW,L of 209 PCDE congeners [71][41]. Based on the number of substituting Cl atoms on the different positions of parent compound diphenyl ether and the number of relative positions for these Cl atoms, a QSPR model was established by the theoretical linear solvation energy relationship (TLSER) method to predict the PL of PCDEs with correlation coefficients R2 of 0.991 [72][42]. An MLR approach was utilized to develop QSPR models to predict the PL of 106 PCDEs based on calculated molecular descriptors [73][43]. The SW,L values of five PCDE congeners were predicted using a PLS method [74][44]. The physicochemical properties predicted from the QSPR models mentioned above are listed in Table S3 [69,71,72,73,74][39][41][42][43][44]. The experimental and predicted results show that logPL, logKow and logSw,l of PCDEs range from −5.97 to −0.27, 4.38 to 8.31 and −12.95 to −4.21, respectively. These physicochemical properties indicate that PCDEs tend to accumulate in environments rich in organic matter, such as soils, sediments and organisms.
3. Environmental Levels
3.1. Water
To the best of outhe researchers' knowledge, only three studies are available on the levels of PCDEs in water. Samples from the contaminated area of Whitby Harbor and a bridge near the entrance to Pringle Creek on the north shore of Lake Ontario were analyzed; 45 PCDE congeners were found in the semi-permeable membrane device (SPMD) at total concentrations of 0.68–7.07 ng/L [11][22]. In China, 15 PCDE congeners were detected in surface water samples from the Nanjing section of the Yangtze River [13][45]. The total concentration ranged from 1150 to 1800 ng/L and 730 to 1300 ng/L during the low- and high-water periods, respectively, with CDE 30 being the dominant congener. In the next study by the same group, the total concentrations of the PCDE congeners ranged from 0.351 to 2.021 ng/L in surface water samples from Chaohu Lake and its eight main tributaries in China, with CDE 30 (20.63%), CDE 28 (9.78%) and CDE 37 (9.52%) as the major congeners [12][34]. In general, PCDEs with less substituted Cl atoms have lower logKow and relatively higher water solubility [41][11]. Therefore, lower chlorinated PCDEs, such as mono-, di- and tri-CDEs are more easily transferred to the aqueous phase than higher chlorinated congeners [12][34]. The presence of PCDEs in water may be associated with surrounding or upstream industrial production and human activities, such as the production and use of chlorophenols, clofibrate, triclosan, bifenox, 2-chlorophenyl N-methylcarbamate and triadimefon [11,12,13,57][22][33][34][45]. Studies showed that CDE 37 and 77 could induce severe oxidative damage in green algae (Scenedesmus obliquus), water flea (Daphnia magna), zebrafish (Danio rerio) and crucian carp (Carassius auratus) at environmentally relevant concentrations [59,75][37][46].
3.2. Sediment and Suspended Particulate Matter
PCDEs tend to accumulate in the sediment compared to water due to their higher hydrophobicity. The pollution of sediment by PCDEs was first reported for Whitby Harbour on the north shore of Lake Ontario in 1981 [76][47]. Subsequently, the environmental exposure of PCDEs has gradually received attention. The mean concentrations of total PCDE congeners in sediments of the contaminated area of Whitby Harbour were between 622 and 1929 ng/g dw in 1995 [11][22]. The average detection concentration of PCDEs in Lake Ontario was 1.30 ng/g dw, which was comparable to that of PCDDs (1.10 ng/g dw) and PCDFs (2.44 ng/g dw) [18][15]. In the sediment of Kymijoki River in Finland, which was highly contaminated by PCDEs due to the intensive production and use activities nearby of chlorophenol in the 19th century, the total concentration of PCDEs was determined in the range of approximately 130 to 554 ng/g dw (50 congeners tested) in 1993 [15][48], 8.79 to 606 ng/g dw (40 congeners tested), except for the reference sediment, in 1997 [16][49], and 85 ng/g dw (nine congeners tested) in 2001 [17][14]. The types and quantities of the measured compounds were different; therefore, it is difficult to judge the changing trend of PCDEs concentration in sediments of the Kymijoki River year by year. In industrially developed areas of eastern China, sediment samples were collected from Chaohu Lake and the Nanjing section of the Yangtze River, where the total concentrations of 15 PCDE congeners were in the range of 0.279–2.47 ng/g dw and 1.24–3.98 ng/g dw, respectively [12,13][34][45]. The level of PCDEs (mean: 1.30 ng/g dw) in the sediments of Chaohu Lake were higher than that of structurally similar polybrominated diphenyl ethers (PBDEs) tested (mean: 0.714 ng/g dw) [77][50], while lower than that of PCBs (mean: 12.07 ng/g dw) [78][51]. In addition to sediments, PCDEs in suspended particulate matter (SPM) of Chaohu Lake were also detected. The result showed that the mean total concentration of PCDEs in SPM was comparable to that in the sediment, which was 1.15 ng/g dw, lower than that of PBDEs (mean: 232.5 ng/g dw). In the SPM of the upper Narragansett Bay, the detected concentrations of tri-CDEs and tetra-CDEs were 0.03 ppt dw and 0.06 ppt dw, respectively, which were lower than that of tri-CDF (0.25 ppt dw) [19][52]. Furthermore, compared with the chlorinated degree of PCDEs in water, PCDEs with more chlorine atoms were more likely to accumulate in sediment and SPM.
3.3. Soils
By contrast, very little information is available on the levels of PCDEs in soils. An earlier study showed that the total concentration of 19 PCDE congeners ranged from <38 to 6800 ng/g dw in soils at 5 contaminated sawmill sites in Sweden [20][53].
3.4. Atmosphere
Only one report to outhe researchers' knowledge has recently showed the levels of PCDEs in the atmosphere. That is, the atmospheric occurrence of six PCDE congeners were investigated over the rural area and the Pacific Ocean near Taiwan and the northern Philippines [21][35]. An elevated mean level of PCDEs was found in the ambient air of the rural area (0.014 pg/m3) compared with that found in the oceanic atmosphere (0.00875 pg/m3). CDE 28 was the predominant congener, accounting for 98.3 and 95.8% of the total PCDEs in the oceanic atmosphere and the ambient air over the land, respectively.
3.5. Biological Organisms
Organisms are susceptible to contamination by PCDEs in the environment due to their lipophilic nature. The presence of PCDEs in organisms was first identified in marine organisms, including clam (Mercenaria mercenaria), mussel (Mytilus edulis) and lobster (Honarus americanus), from Narragansett Bay in the United States [19][52]. PCDEs were also detected in freshwater fish in the North American Great Lakes. The total concentration of 28 monitored PCDE congeners ranged from 24 to 891 ng/g lw in lake trout (Sulvelinus namaycush) and walleye (Stizostedion vitreum vitreum) collected from the Great Lakes on a whole-fish basis [29][54]. Penta-, hexa- and hepta-chlorinated congeners were the most abundant homologue groups, representing approximately 80 to 90% of the total concentrations. In another study, the occurrence of 15 PCDE congeners was examined in whole fish samples of common carp (Cyprinus carpio) and northern pike (Esox lucius) caught from Whitby Harbour on the north shore of Lake Ontario [28][55]. The total levels of PCDEs varied from 768 to 14,005 ng/g ww, well above the detected concentrations of PCDFs (58–254 pg/g ww). In a later investigation on 8 fish species, including common shiner (Notropis cornutus), rosyface shiners (Notropis rubellus), spottail shiner (Notropis hudsonius), pumpkinseed (Lepomis gibbosus), yellow perch (Perca flavescens), brown bullhead (Ameiurus nebulosus), white sucker (Catostomus commersoni) and northern pike (Esox lucius), collected also from Whitby Harbour, the total lipid-normalized concentrations of 45 PCDE congeners in muscle samples for each species ranged from 100 to 2857 ng/g, 23231 to 43,231 ng/g, 20,706 to 96,529 ng/g, 30,417 to 68,250 ng/g, 4200 to 130,333 ng/g, 7538 to 213,231 ng/g, 16,714 to 174,571 and 21,000 to 47,000 ng/g, respectively [11][22]. CDE 99, 153 and 154 were the dominant congeners, and CDE 47, 74, 100, 118, 163, 182 and 184 were also significant. In addition to fish in inland lakes and coastal waters, PCDEs were also indirectly detected in deep sea fish through investigating levels of 106 PCDE congeners in 2 cod liver oils made from North Atlantic deep sea fish [9]. The total PCDE levels were 49 and 659 ng/g lw, respectively. These studies reflect the common presence of PCDEs in organisms in both marine and freshwater environments.
PCDEs have also been detected in organisms in other countries and regions. In oligochaete worm (Lumbriculus variegatus), chironomids and northern pike (Esox lucius) collected from sampling sites in the Kymijoki River in Finland, located downstream of an adjacent Ky-5 (which was a chlorophenol wood preservative) production plant, the total concentrations of 40 or 50 PCDE congeners were detected ranging from 215 to 1325 ng/g lw, 0 to 1200 ng/g lw and 677 to 706 ng/g lw, respectively [15,16][48][49]. The patterns of PCDE levels in these organisms were similar and resembled that in the sediments collected at the same sampling sites, and these dominant congeners were also abundant in Ky-5 as well. The major PCDE congeners detected in salmon from the Tenojoki Rive, Lake Saimaa and the Simojoki River in Finland were also similar and abundant in Ky-5 too [30][56]. Moreover, the congener patterns appear to be similar to those detected in Whitby Harbour fish [11][22]. It indicates that PCDEs contamination in the two regions may be attributed to the production or use of Ky-5 there.
PCDEs in organisms are acquired not only by bioconcentration from the ambient environment, but also by biomagnification throughout the food chain. PCDEs have been detected in birds and mammals that eat fish and other aquatic organisms. For example, eggs of fish-eating birds, including common tern (Sterna hirundo), black skimmer (Rynchops niger) and bald eagle (Haliaeetus leucocephalus) from Rhode Island, Louisiana, Michigan and Ohio were examined, and it was found that the total concentrations of three PCDE congeners tested ranged from 11 to 900 ng/g ww [24][57]. The high concentrations of PCDEs (sum of 7 congeners) were also found in eggs of black-crowned night herons (Nycticorax nycticorax) from Tianmu Lake and whiskered terns (Chlidonias hybrid) from East Tai Lake in China with levels ranging from 11 to 450 ng/g lw and 15 to 700 ng/g lw, respectively [26][58]. They were well above the detected total concentrations of PCDD/Fs of 0.38–19 and 2.6–33 ng/g lw in the two birds, respectively. In an investigation within the Baltic Sea area as the most polluted brackish water area in the world, the concentrations of individual PCDE congeners were detected ranging from <3 to 79 ng/g lw in eggs of black guillemots (Cepphus grylle L.) and from <5 to 13,000 ng/g lw in breast muscle of white-tailed sea eagles (Hallaeetus albicilla L.) as a top predator of the Baltic food chain [25][59]. The total concentrations of the 50 tested PCDE congeners varied from 233 to 354 ng/g lw and 1027 to 50,924 ng/g lw, respectively. They were also significantly higher than those of PCDD/Fs, i.e., 3.9–4.0 ng/g lw in black guillemots (Cepphus grylle L.) and 1.6–133 ng/g lw in white-tailed sea eagles (Hallaeetus albicilla L.). In mammals, such as seals, high levels of PCDEs were also detected. The contents of 50 individual congeners ranged from <0.3 to 62 ng/g lw in blubber of ringed seals (Phoca hispida botnica) and grey seals (Halichoerus grypus) from the Gulf of Finland in the Baltic Sea with the total concentrations of 39.9–373.9 ng/g lw [14][60]. PCDEs were at similar levels in the seal blubber compared to fish captured here and from the Kymijoki River that finally flows into the Gulf of Finland [16,27][49][61]. In blubber samples of a Baikal seal (Phoca sibirica) from Lake Baikal in East Siberia of Russia and several ringed seals (Phoca hispida saimensis) from Lake Saimaa in Southeast Finland, the total concentrations of the 50 congeners were found to be 60 ng/g lw and 217–459 ng/g lw, respectively [15][48]. In blubber samples of harbor seals (Phoca vitulina) captured from the Salish Sea in north–western North America, lower total contents of PCDE congeners (6.5–21 ng/g lw; sum of 46 congeners) were measured, which might be due to light PCDEs contamination in North America [33][62]. Furthermore, studies have demonstrated the presence of PCDEs in human adipose tissue. Tetra- to deca-CDE congeners in human adipose tissue collected from Canadian municipalities were analyzed. CDE 206 and 209 were found to be in the range of 0.1–2.9 ng/g lw, and the mean level of CDE 206 in males was greater than that in females [34][63]. Six hexa- to deca-CDE congeners were also detected in human adipose tissue from the USA, where the predominant congener was CDE 206 with concentrations ranging from 0.6 to 1.4 ng/g lw [35][64]. In addition, it was reported that the concentrations of 50 individual PCDE congeners varied between <0.5 and 7.9 ng/g lw in Finnish human adipose tissue, which were comparable to the levels of PCDD and PCDF congeners (<5 to 7700 pg/g lw) [35][64]. The main origin of PCDEs found in humans may be contaminated food. Human exposure to PCDEs through the diet was first reported in Catalonia (Spain) in 2004 [36][65]. PCDEs were detected in a number of foodstuffs available in the local market. The total PCDE concentrations in fresh hake (Rexea solandri), fresh sardine (Sardina pilchardus), mussels and tinned fish were 45.9–707, 400–2707, 59.8–107 and 3.3–71.9 pg/g ww, respectively. Total dietary intake of PCDEs through fish and shellfish was estimated to be 38 ng/day by a standard male adult of 70 kg body weight and aged between 20 and 65 years in Catalonia (Spain), which was slightly higher than PBDEs of approximately 31 ng/day. Moreover, PCDE intake was always higher in males than in females for people under 45 years old due to a greater food intake by males. In a subsequent study by the same research group, the concentrations of PCDEs were determined in 14 edible marine species widely consumed by the population of Catalonia (Spain) [38][66]. The highest PCDE levels (pg/g ww) were found in red mullet (Mullus barbatus; 7088) followed by sardine (Sardina pilchardus; 1829), anchovy (1606), tuna (Scombridae gen. sp.; 1292) and mackerel (1031). Children aged 4–9 years (boys 0.88 ng/kg/day and girls 0.73 ng/kg/day) showed the highest PCDE intake when judged by the average body weight [79][67]. Dietary intake of PCDEs in athletes was also evaluated [37][68]. In general, sportsmen and sportswomen showed a lower daily dietary intake than the general population due to ingesting lower amounts of fish and seafood. In another survey of PCDEs in foodstuffs in Catalonia (Spain) in 2006, the dietary intake of PCDEs was 51.68 ng/day for a standard male adult of 70 kg body weight, increasing by 26% compared to the previous survey (41 ng/day) in 2000, with fish and seafood being the main contributors to this increase [36,39][65][69]. In addition, the influence of different cooking processes including frying, grilling, roasting and boiling on the levels of PCDEs in various foodstuffs was evaluated. Studies showed that almost all cooking processes enhanced the total PCDEs levels in fish and meat samples [40,80][23][70]. Detailed information about the levels of PCDEs in various environmental media and biota reported previously is provided in Figure 3.
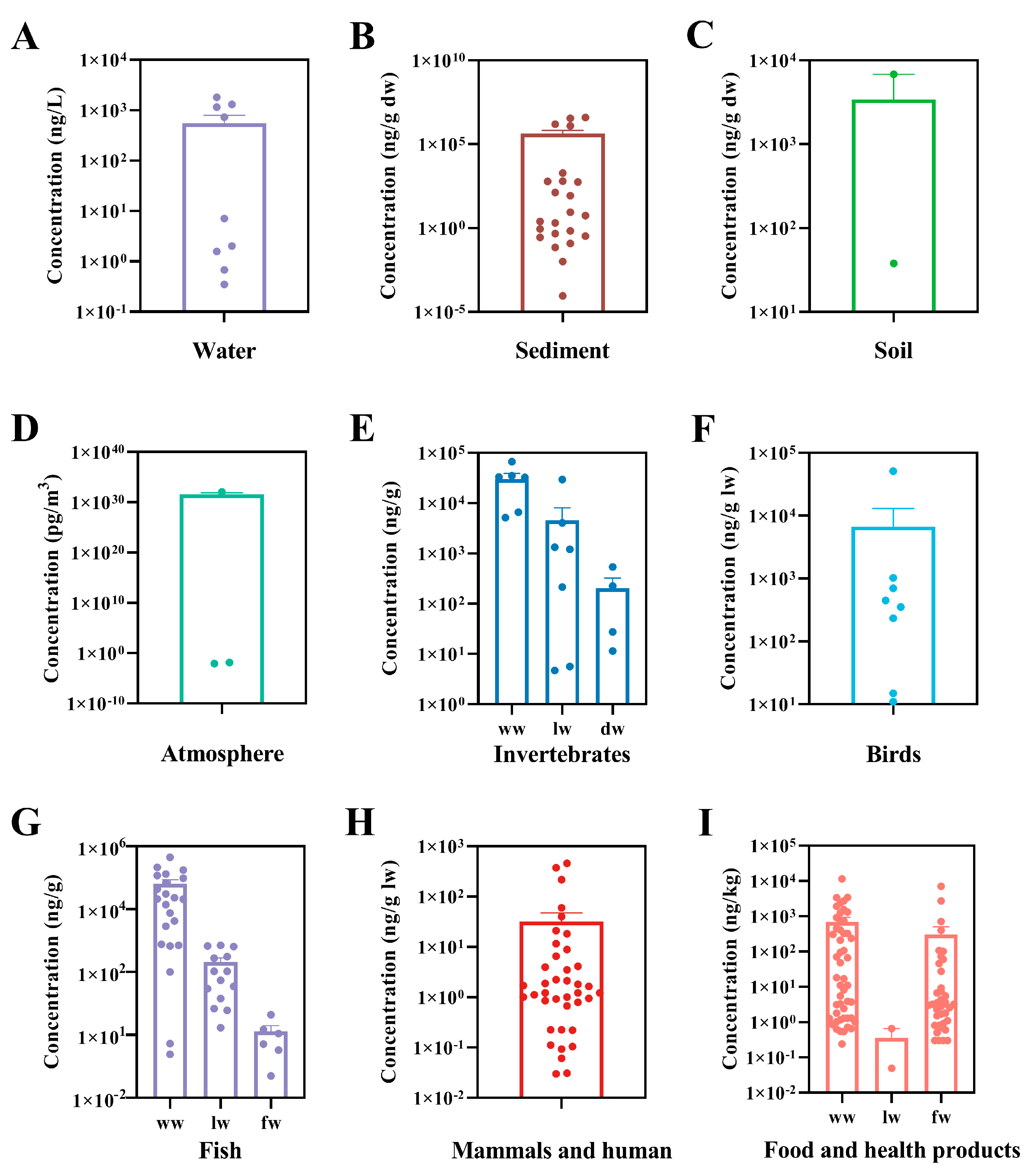
Figure 3. Concentrations of PCDEs detected in water (A), sediment (B), soil (C), atmosphere (D), invertebrates (E), birds (F), fish (G), mammals and human (H) and food and health products (I).
References
- Koistinen, J. Polychlorinated Diphenyl Ethers (PCDE); Springer: Berlin/Heidelberg, Germany, 2000; Volume 3, pp. 157–201.
- Nevalainen, T.J.; Rissanen, K. AM1 and single-crystal X-ray diffraction study of the conformational properties of chlorinated diphenyl ethers. J. Chem. Soc. Perkin Trans. 2 1994, 271–279.
- Becker, M.; Phillips, T.; Safe, S. Polychlorinated diphenyl ethers—A review. Toxicol. Environ. Chem. 1991, 33, 189–200.
- Sundström, G.; Hutzinger, O. The synthesis of chlorinated diphenyl ethers. Chemosphere 1976, 5, 305–312.
- Albro, P.W.; Parker, C.E. General approach to the fractionation and class determination of complex mixtures of chlorinated aromatic compounds. J. Chromatogr. A 1980, 197, 155–169.
- Ningbo Inno Pharmchem Co., Ltd. Intermediates of Difenoconazole CAS: 119446-68-3. Available online: https://www.tfindia.com/34-dichlorodiphenyl-ether/ (accessed on 1 February 2023).
- Domingo, J.L. Polychlorinated diphenyl ethers (PCDEs): Environmental levels, toxicity and human exposure: A review of the published literature. Environ. Int. 2006, 32, 121–127.
- Nilsson, C.A.; Renberg, L. Further studies on impurities in chlorophenols. J. Chromatogr. A 1974, 89, 325–333.
- Kurz, J.; Ballschmiter, K. Isomer-specific determination of 79 polychlorinated diphenyl ethers (PCDE) in cod liver oils, chlorophenols and in a fly ash. Fresenius’ J. Anal. Chem. 1995, 351, 98–109.
- Yang, J.S.; Lin, S.L.; Lin, T.C.; Wu, Y.L.; Wang, L.C.; Chang-Chien, G.P. Emissions of polychlorinated diphenyl ethers from a municipal solid waste incinerator during the start-up operation. J. Hazard. Mater. 2015, 299, 206–214.
- Kurz, J.; Ballschmiter, K. Vapour pressures, aqueous solubilities, Henry’s law constants, partition coefficients between gas/water (Kgw), N-octanol/water (Kow) and gas/N-octanol (Kgo) of 106 polychlorinated diphenyl ethers (PCDE). Chemosphere 1999, 38, 573–586.
- Koistinen, J.; Mussalo-Rauhamaa, H.; Paasivirta, J. Polychlorinated diphenyl ethers, dibenzo-p-dioxins and dibenzofurans in finnish human tissues compared to environmental samples. Chemosphere 1995, 31, 4259–4271.
- Sinkkonen, S.; Paasivirta, J. Polychlorinated organic compounds in the Arctic cod liver: Trends and profiles. Chemosphere 2000, 40, 619–626.
- Sormunen, A.J.; Koistinen, J.; Leppänen, M.T.; Kukkonen, J.V.K. Desorption of sediment-associated polychlorinated dibenzo-p-dioxins, dibenzofurans, diphenyl ethers and hydroxydiphenyl ethers from contaminated sediment. Chemosphere 2008, 72, 1–7.
- Li, A.; Guo, J.; Li, Z.; Lin, T.; Zhou, S.; He, H.; Ranansinghe, P.; Sturchio, N.C.; Rockne, K.J.; Giesy, J.P. Legacy polychlorinated organic pollutants in the sediment of the Great Lakes. J. Great Lakes Res. 2018, 44, 682–692.
- Niimi, A.J. Biological half-lives of chlorinated diphenyl ethers in rainbow trout (Salmo gairdneri). Aquat. Toxicol. 1986, 9, 105–116.
- Opperhuizen, A.; Voors, P.I. Bioconcentration kinetics of 2,4,5- tri- and 3,3′,4,4′-tetrachlorobiphenyl and 2,4,5- tri- and 3,3′,4,4′-tetrachlorodiphenylether in fish. Chemosphere 1987, 16, 2379–2388.
- Chui, Y.C.; Addison, R.F.; Law, F.C. Acute toxicity and toxicokinetics of chlorinated diphenyl ethers in trout. Xenobiotica 1990, 20, 489–499.
- Zitko, V.; Carson, W.G. Uptake and excretion of chlorinated diphenyl ethers and brominated toluenes by fish. Chemosphere 1977, 6, 293–301.
- Neely, W.B.; Branson, D.R.; Blau, G.E. Partition coefficient to measure bioconcentration potential of organic chemicals in fish. Environ. Sci. Technol. 1974, 8, 1113–1115.
- Lyytikäinen, M.; Hirva, P.; Minkkinen, P.; Hämäläinen, H.; Rantalainen, A.L.; Mikkelson, P.; Paasivirta, J.; Kukkonen, J.V.K. Bioavailability of Sediment-Associated PCDD/Fs and PCDEs: Relative Importance of Contaminant and Sediment Characteristics and Biological Factors. Environ. Sci. Technol. 2003, 37, 3926–3934.
- Villeneuve, J.Y.; Niimi, A.J.; Metcalfe, C.D. Distribution and Bioaccumulation of Chlorinated Diphenyl Ethers in a Contaminated Embayment of Lake Ontario. J. Great Lakes Res. 1999, 25, 760–771.
- Perelló, G.; Martí-Cid, R.; Castell, V.; Llobet, J.M.; Domingo, J.L. Influence of various cooking processes on the concentrations of PCDD/PCDFs, PCBs and PCDEs in foods. Food Control 2010, 21, 178–185.
- Qin, L.; Liu, F.; Liu, H.; Wei, Z.; Sun, P.; Wang, Z. Evaluation of HODE-15, FDE-15, CDE-15, and BDE-15 toxicity on adult and embryonic zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2014, 21, 14047–14057.
- Metcalfe, C.D.; Metcalfe, T.L.; Cormier, J.A.; Huestis, S.Y.; Niimi, A.J. Early life-stage mortalities of Japanese medaka (Oryzias latipes) exposed to polychlorinated diphenyl ethers. Environ. Toxicol. Chem. 1997, 16, 1749–1754.
- Zhang, X.; Feng, M.; Liu, F.; Qin, L.; Qu, R.; Li, D.; Wang, Z. Subacute oral toxicity of BDE-15, CDE-15, and HODE-15 in ICR male mice: Assessing effects on hepatic oxidative stress and metals status and ascertaining the protective role of vitamin E. Environ. Sci. Pollut. Res. Int. 2014, 21, 1924–1935.
- Rosiak, K.; Li, M.H.; Degitz, S.J.; Skalla, D.W.; Chu, I.; Francis, B.M. Maternal and developmental toxicity of polychlorinated diphenyl ethers (PCDEs) in Swiss-Webster mice and Sprague-Dawley rats. Toxicology 1997, 121, 191–204.
- Rosiak, K.L.; Seo, B.W.; Chu, I.; Francis, B.M. Effects of maternal exposure to chlorinated diphenyl ethers on thyroid hormone concentrations in maternal and juvenile rats. J. Environ. Sci. Health Part B 1997, 32, 377–393.
- Harper, N.; Howie, L.; Connor, K.; Arellano, L.; Craig, A.; Dickerson, R.; Safe, S. Immunosuppressive and Monooxygenase Induction Activities of Highly Chlorinated Diphenyl Ether Congeners in C57BL/6 and DBA/2 Mice. Fundam. Appl. Toxicol. 1993, 20, 496–502.
- Safe, S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 1990, 21, 51–88.
- Howie, L.; Dickerson, R.; Davis, D.; Safe, S. Immunosuppressive and monooxygenase induction activities of polychlorinated diphenyl ether congeners in C57BL6N mice: Quantitative structure-activity relationships. Toxicol. Appl. Pharmacol. 1990, 105, 254–263.
- Wu, E.M.Y.; Wang, L.C.; Lin, S.L.; Chang, C.G.P. Validation and characterization of persistent organic pollutant emissions from stack flue gases of an electric arc furnace by using a long-term sampling system (AMESA®). Aerosol. Air Qual. Res. 2014, 14, 185–196.
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213.
- Zhang, X.; Wang, T.; Gao, L.; Feng, M.; Qin, L.; Shi, J.; Cheng, D. Polychlorinated diphenyl ethers (PCDEs) in surface sediments, suspended particulate matter (SPM) and surface water of Chaohu Lake, China. Environ. Pollut. 2018, 241, 441–450.
- Chao, H.R.; Lin, D.Y.; Chen, K.Y.; Gou, Y.Y.; Chiou, T.H.; Lee, W.J.; Chen, S.J.; Wang, L.C. Atmospheric concentrations of persistent organic pollutants over the Pacific Ocean near southern Taiwan and the northern Philippines. Sci. Total Environ. 2014, 491–492, 51–59.
- Zhang, X.S.; Xiong, W.L.; Wu, Q.X.; Nian, K.N.; Pan, X.X.; Doug, C. Bioaccumulation, trophic transfers and biotransformation of polychlorinated diphenyl ethers in an indoor simulated aquatic food chain. Environ. Sci. Technol. 2023; submitted.
- Yang, W.; Huang, X.; Wu, Q.; Shi, J.; Zhang, X.; Ouyang, L.; Crump, D.; Zhang, X.; Zhang, R. Acute toxicity of polychlorinated diphenyl ethers (PCDEs) in three model aquatic organisms (Scenedesmus obliquus, Daphnia magna, and Danio rerio) of different trophic levels. Sci. Total Environ. 2022, 805, 150366.
- Kurz, J.; Ballschmiter, K. Relationship between structure and retention of polychlorinated diphenyl ethers (PCDE) in HRGC in comparison with other groups of halogenated aromatic compounds. Fresenius’ J. Anal. Chem. 1994, 349, 533–537.
- Yang, P.; Chen, J.; Chen, S.; Yuan, X.; Schramm, K.W.; Kettrup, A. QSPR models for physicochemical properties of polychlorinated diphenyl ethers. Sci. Total Environ. 2003, 305, 65–76.
- Xu, H.Y.; Zou, J.W.; Hu, G.X.; Wang, W. QSPR/QSAR models for prediction of the physico-chemical properties and biological activity of polychlorinated diphenyl ethers (PCDEs). Chemosphere 2010, 80, 665–670.
- Sun, L.; Zhou, L.; Yu, Y.; Lan, Y.; Li, Z. QSPR study of polychlorinated diphenyl ethers by molecular electronegativity distance vector (MEDV-4). Chemosphere 2007, 66, 1039–1051.
- Zeng, X.; Wang, Z.; Ge, Z.; Liu, H. Quantitative structure–property relationships for predicting subcooled liquid vapor pressure (PL) of 209 polychlorinated diphenyl ethers (PCDEs) by DFT and the position of Cl substitution (PCS) methods. Atmos. Environ. 2007, 41, 3590–3603.
- Yuan, Y.; Sun, Y.; Wang, D.; Liu, R.; Gu, S.; Liang, G.; Xu, J. Quantitative structure-property relationship study of liquid vapor pressures for polychlorinated diphenyl ethers. Fluid Phase Equilib. 2015, 391, 31–38.
- Xiao, F.; Gulliver, J.S.; Simcik, M.F. Predicting aqueous solubility of environmentally relevant compounds from molecular features: A simple but highly effective four-dimensional model based on Project to Latent Structures. Water Res. 2013, 47, 5362–5370.
- Qin, L.; Feng, M.; Zhang, X.; Wang, L.; Wang, Z. Occurrence of polychlorinated diphenyl ethers in Nanjing section of the Yangtze River: Level and distribution pattern. Environ. Sci. Pollut. Res. 2015, 22, 9224–9232.
- Cheng, D.; Cao, K.; Wang, T.; Zhang, X.; Feng, M.; Liu, H. Evaluation of the oxidative stress in liver of crucian carp (Carassius auratus) exposed to 3,4,4′-tri-CDE, 2-MeO-3′,4,4′-tri-CDE, and 2-HO-3′,4,4′-tri-CDE. Environ. Sci. Pollut. Res. Int. 2019, 26, 5164–5175.
- Coburn, J.A.; Comba, M. Identification of Polychlorinated Diphenyl Ethers in Whitby Harbour Sediments; Association of Analytical Chemist’s, Spring Workshop: Ottawa, ON, Canada, 1981.
- Koistinen, J.; Paasivirta, J.; Suonpera, M.; Hyvarinen, H. Contamination of Pike and Sediment from the Kymijoki River by PCDEs, PCDDs, and PCDFs: Contents and Patterns Compared to Pike and Sediment from the Bothnian Bay and Seals from Lake Saimaa. Environ. Sci. Technol. 1995, 29, 2541–2547.
- Lyytikäinen, M.; Rantalainen, A.L.; Mikkelson, P.; Hämäläinen, H.; Paasivirta, J.; Kukkonen, J. Similarities in bioaccumulation patterns of polychlorinated dibenzo-p-dioxins and furans and polychlorinated diphenyl ethers in laboratory-exposed oligochaetes and semipermeable membrane devices and in field-collected chironomids. Toxicol Environ. Chem. SETAC 2003, 22, 2405–2415.
- He, W.; Qin, N.; Kong, X.; Liu, W.; He, Q.; Ouyang, H.; Wang, Q.; Yang, B.; Yang, C.; Jiang, Y.; et al. Polybrominated diphenyl ethers (PBDEs) in the surface sediments and suspended particulate matter (SPM) from Lake Chaohu, a large shallow Chinese lake. Sci. Total Environ. 2013, 463–464, 1163–1173.
- Huo, S.; Li, C.; Xi, B.; Yu, Z.; Yeager, K.M.; Wu, F. Historical record of polychlorinated biphenyls (PCBs) and special occurrence of PCB 209 in a shallow fresh-water lake from eastern China. Chemosphere 2017, 184, 832–840.
- Lake, J.L.; Rogerson, P.F.; Norwood, C.B. A polychlorinated dibenzofuran and related compounds in an estuarine ecosystem. Environ. Sci. Technol. 1981, 15, 549–553.
- Persson, Y.; Lundstedt, S.; Öberg, L.; Tysklind, M. Levels of chlorinated compounds (CPs, PCPPs, PCDEs, PCDFs and PCDDs) in soils at contaminated sawmill sites in Sweden. Chemosphere 2007, 66, 234–242.
- Niimi, A.J.; Huestis, S.Y.; Metcalfe, C.D. Chlorinated diphenyl ethers in Great Lakes fish and their environmental implication. Environ. Toxicol. Chem. 1994, 13, 1133–1138.
- Huestis, S.Y.; Sergeant, D.B. Removal of chlorinated diphenyl ether interferences for analyses of PCDDs and PCDFs in fish. Chemosphere 1992, 24, 537–545.
- Koistinen, J.; Vuorinen, P.J.; Paasivirta, J. Contents and origin of polychlorinated diphenyl ethers (PCDE) in salmon from the Baltic Sea, Lake Saimaa and the Tenojoki river in Finland. Chemosphere 1993, 27, 2365–2380.
- Stafford, C.J. Halogenated diphenyl ethers identified in avian tissues and eggs by GC/MS. Chemosphere 1983, 12, 1487–1495.
- Zhou, Y.; Yin, G.; Asplund, L.; Stewart, K.; Rantakokko, P.; Bignert, A.; Ruokojärvi, P.; Kiviranta, H.; Qiu, Y.; Ma, Z.; et al. Human exposure to PCDDs and their precursors from heron and tern eggs in the Yangtze River Delta indicate PCP origin. Environ. Pollut. 2017, 225, 184–192.
- Koistinen, J.; Koivusaari, J.; Nuuja, I.; Paasivirta, J. PCDEs, PCBs, PCDDs AND PCDFs in black guillemots and white-tailed sea eagles from the Baltic Sea. Chemosphere 1995, 30, 1671–1684.
- Koistinen, J.; Stenman, O.; Haahti, H.; Suonperä, M.; Paasivirta, J. Polychlorinated diphenyl ethers, dibenzo-p-dioxins, dibenzofurans and biphenyls in seals and sediment from the gulf of finland. Chemosphere 1997, 35, 1249–1269.
- Koistinen, J.; Paasivirta, J.; Lahtiperä, M. Bioaccumulation of dioxins, coplanar PCBs, PCDEs, HxCNs, R-PCNs, R-PCPHs and R-PCBBs in fish from a pulp-mill recipient watercourse. Chemosphere 1993, 27, 149–156.
- Ross, P.S.; Noël, M.; Lambourn, D.; Dangerfield, N.; Calambokidis, J.; Jeffries, S. Declining concentrations of persistent PCBs, PBDEs, PCDEs, and PCNs in harbor seals (Phoca vitulina) from the Salish Sea. Prog. Oceanogr. 2013, 115, 160–170.
- Williams, D.T.; Kennedy, B.; LeBel, G.L. Chlorinated diphenyl ethers in human adipose tissue. Part 2. Chemosphere 1991, 23, 601–608.
- Stanley, J.S.; Cramer, P.H.; Thornburg, K.R.; Remmers, J.C.; Breen, J.J.; Schwemberger, J. Mass spectral confirmation of chlorinated and brominated diphenylethers in human adipose tissues. Chemosphere 1991, 23, 1185–1195.
- Bocio, A.; Llobet, J.M.; Domingo, J.L. Human Exposure to Polychlorinated Diphenyl Ethers through the Diet in Catalonia, Spain. J. Agric. Food Chem. 2004, 52, 1769–1772.
- Domingo, J.L.; Bocio, A.; Falcó, G.; Llobett, J.M. Exposure to PBDEs and PCDEs associated with the consumption of edible marine species. Environ. Sci. Technol. 2006, 40, 4394–4399.
- Martí-Cid, R.; Bocio, A.; Llobet, J.M.; Domingo, J.L. Intake of chemical contaminants through fish and seafood consumption by children of Catalonia, Spain: Health risks. Food Chem. Toxicol. 2007, 45, 1968–1974.
- Falcó, G.; Bocio, A.; Llobet, J.M.; Domingo, J.L. Health risks of dietary intake of environmental pollutants by elite sportsmen and sportswomen. Food Chem. Toxicol. 2005, 43, 1713–1721.
- Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Human Exposure to Polychlorinated Naphthalenes and Polychlorinated Diphenyl Ethers from Foods in Catalonia, Spain: Temporal Trend. Environ. Sci. Technol. 2008, 42, 4195–4201.
- Domingo, J.L. Nutrients and Chemical Pollutants in Fish and Shellfish. Balancing Health Benefits and Risks of Regular Fish Consumption. Crit. Rev. Food Sci. Nutr. 2016, 56, 979–988.
More
