Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Paweł Rasmus and Version 2 by Peter Tang.
Depression has a multifactorial etiology comprising family history and unemployment. Antioxidant supplementation has been found to combat various stress-induced psychiatric disorders, including depression and anxiety. A growing body of evidence indicates that carotenoids have both antioxidant and anti-inflammatory. Studies also suggest that poor dietary intake, particularly low intakes of fruit and vegetables and high intakes of fast food and other convenience foods, may increase the risk of developing depression. Thus, dietary interventions have the potential to help mitigate the risk of mental health decline in both the general population and those with mood disorders.
- depression
- carotenoids
- mental health
- nutrition
1. Introduction
Some of the most common noncommunicable illnesses are psychiatric disorders such as depression; together, these are responsible for 14.3% of global fatalities. It is estimated that 322 million individuals live with depression globally and that depression is involved in 50–70% of suicides [1]. The World Health Organization predicts depression will become the second most prevalent disorder after ischemic heart disease [2]. Depression is most commonly manifested as hopelessness, disturbances in sleep or appetite, and avoiding social activity, and it also acts as an independent predictor of the beginning of somatic illness [3]. Studies have shown that people with depression have a higher risk of developing lifestyle disorders such as cardiovascular disease, obesity, and diabetes, as well as cancer; it has also been associated with cognitive impairment [4][5][6][7][8][4,5,6,7,8]. However, while it is impossible to avoid some of the most common risk factors, such as family history, poverty, and unemployment [9][10][9,10], it is possible to modify others, such as a sedentary lifestyle [11], cigarette smoking [12] and alcohol consumption [13].
Depression has a multifactorial etiology comprising functional deficits in monoamine, such as serotonin, and disturbances in the brain’s hypothalamic–pituitary–adrenal (HPA) axis [14]. In addition, emotional disorders such as anxiety and depression are often associated with a reduction in the activity of antioxidant enzymes in the HPA axis. HPA axis hyperactivity is often observed in cases of depression; this correlates with an increase in glucocorticoid concentration, resulting in the development of oxidative stress and glutamatergic excitotoxicity and thus the death of nerve cells, especially in the hippocampus, the key region related to the regulation of mood [15][16][17][18][19][20][21][15,16,17,18,19,20,21]. Also, chronic psychological stress, known to contribute to the development of depression, causes structural changes in the hippocampus associated with the regulation of cognition [22].
It should be emphasized that although there are many theories of depression development, its accurate pathomechanism is still not fully known. Depression is widely believed to be strongly influenced by immuno-inflammatory mechanisms [23][24][25][23,24,25]. Patients with depression tend to demonstrate significantly elevated levels of various chemokines (CCL2, CXCL10), and pro-inflammatory cytokines, such as interleukin (IL)−1β, IL-6, and tumor necrosis factor (TNF) [26][27][28][29][30][26,27,28,29,30]. Some studies found patients with depression to have increased levels of C reactive protein (CRP), an important inflammatory marker [31][32][31,32]. Current literature indicates a strong two-way association between the development of inflammation and psychiatric disorders, including depression. It should also be borne in mind that depression is related to an exacerbation of behaviors associated with the development of inflammation, such as nutritional deficiencies/poor eating habits, and addiction to psychoactive substances, such as alcohol, drugs, or smoking [33]. In addition, there is evidence that commensal gut microorganisms, which comprise the gut microbiota, may play an important role in the etiopathogenesis of depression via the gut-brain axis [34]. The gut-brain axis is a two-way communication pathway between the central nervous system and the gut. This communication occurs via hormonal, neurological, and immunological signaling systems, as well as gut microbe metabolites, which trigger changes in neurotransmission, neuroinflammation, and behavior [35][36][35,36]. Disturbances in the composition, quality, and functioning of the intestinal microbiota (intestinal dysbiosis) are correlated with some neuropsychiatric disorders, especially depression. Numerous studies have investigated the potential impact of gut microbiota on the onset of depression [37][38][39][40][41][42][43][44][45][37,38,39,40,41,42,43,44,45].
2. The Influence of Diet on the Development and Course of Mood Disorders
A number of studies indicate that an improperly-balanced diet is one of the elements associated with the development of depression and anxiety [46][47][46,47]. Recent years have seen a growth in interest in the relationship between nutrients and depression, particularly folic acid, vitamin D, and magnesium [48][49][50][48,49,50]. Mikkelsen et al. (2016) demonstrated a relationship between vitamin B deficiency, including B1, B3, B6, B9, B12, and depression [51][52][51,52]. Studies have also shown that a higher level of depression in adolescents is associated with irregular meals [53]. Research indicates that fat-soluble nutrients, such as vitamin E, protect against nerve damage, and low dietary intake is linked to mood changes and depression [54]. It is believed that carotenoid-cleaving enzymes, which take part in the metabolism of carotenoids, play a key role in depression. It should be pointed out that apocarotenoids are formed due to the oxidative breakdown of carotenoids catalyzed by carotenoid oxygenases. Apocarotenoids are, inter alia, retinal, retinol, retinoic acid, and abscisic acid. Some studies describe that retinoic acid, the active form of vitamin A, causes hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and leads to the development of typical depressive behaviors. In addition, it has been shown that retinoic acid may cause suicide in some susceptible individuals [55][56][55,56]. A cross-sectional study of people of normal BMI by Nguyen et al. (2017) found significantly lower intake of β-carotene equivalent and vitamins C, E, B1, B3, B6, B9, and B5 in those with depressive symptoms [54]. It should be noted that vitamin C insufficiency has also been linked to an increased risk of depression symptoms [54]. Additionally, randomized, placebo-controlled clinical trials have found vitamin C to improve mood and lower the severity of depression in patients [57][58][57,58]. Interestingly, however, vitamin C supplementation appeared to have no effect on the depression score in these people [59]. A study conducted among elderly Japanese people showed that the consumption of carotene and vitamin C is associated with less severe depression symptoms [60]. Lower carotenoid concentrations may also reflect unhealthy eating patterns associated with overweight and obesity, which have been linked to an increased risk of depression by inflammation or dysregulation of the HPA axis [5][61][62][5,61,62]. Previous studies have shown that some dietary factors, such as fruit and vegetables, fish, dietary fiber, and some macro- and microelements, may play an important, protective role in the development of depression through their antioxidant and/or anti-inflammatory properties [63][64][65][66][63,64,65,66]. It is important to note that carotenoids are also known for their antioxidant activity and anti-inflammatory properties [67][68][67,68]. Additionally, research shows that depression leads to the development of diseases such as cardiovascular diseases, insulin resistance, metabolic syndrome, and obesity [69][70][71][69,70,71]. These data support the hypothesis that inflammation and oxidative stress may be involved in the pathophysiology of this disorder. Considering that carotenoids have both antioxidant and anti-inflammatory effects, it is expected that they may exert an antidepressant effect.3. The Role of Stress in Unipolar Mood Disorder Pathology
The results of animal studies indicate that psychological stress can increase the level of lipid peroxidation, a significant source of the cell damage caused by reactive oxygen species (ROS) [72], and impair antioxidant protection in the plasma [73][74][73,74]. Due to its high oxygen consumption and relatively weak antioxidant defense, the brain is particularly susceptible to oxidative damage, which may increase the likelihood of developing depressive episodes. Therefore, oxidative stress, caused by an imbalance between antioxidants and prooxidants, may play a key role in the remission and chronic course of depressive disorder [75][76][75,76]. Patients with depression have been found to have a significantly lower mean intake of α-carotene compared to healthy subjects [77][83]. In addition, depression has been associated with lowered antioxidant levels, as evidenced by low levels of carotenoids and antioxidant enzymes [76][78][79][80][76,77,84,85]. Black et al. (2016) found reduced levels of carotenoids such as zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, and β-carotene to be associated with an increase in depression symptoms. Most importantly, this relationship persisted after controlling for diet quality; as carotenoids are only acquired through the dietary route, diet could be considered a significant confounder [3]. Beydoun et al. (2013) report that among the studied carotenoids, β-carotene, lutein, and zeaxanthin levels were inversely related to the incidence of depressive symptoms among US adults [80][85]. Interestingly, these studies suggest that common genetic factors may influence the relationship between low carotenoid levels and depression: the presence of SNPs associated with low β-cryptoxanthin levels may also influence the occurrence of depression [81][86]. In turn, Tsubi et al. [72] and Nouri et al. (2020) [82][96] found no correlation between the serum level of lycopene and depressive symptoms. Zhang et al. (2016) report that seven days of pretreatment with 60 mg/kg lycopene could reverse LPS-induced depressive behavior in mice based on the tail suspension test and the forced swim test [83][97]. In turn, a mechanistic study by Lin et al. (2014) found that three-day treatment with 10 mg/kg lycopene reverses the LPS-induced increase in serum TNF and IL-6 concentrations and IL-1β levels in the hippocampus [84][98]; in addition, pretreatment with 5, 10, or 20 M lycopene inhibited LPS-induced production of cyclooxygenase-2, inducible nitric oxide synthase, and IL-6 in primary cultured microglia via the activation of heme oxygenase-1 [84][98]. There is a possibility that lycopene supplementation may help maintain cellular homeostasis by restoring normal cell cytokines levels turn. These results suggest that inhibiting neuroinflammation may be a key factor in the antidepressant effects of lycopene.4. Oxidative Stress and Antioxidants in the Course of Mood Disorders
Oxidative stress occurs as a result of an imbalance between the build-up of reactive oxygen species (ROS) or reactive nitrogen species (RNS) and their removal. ROS levels are believed to increase due to various environmental features such as tobacco smoke, ionizing, UV radiation, and by the initiation of cell receptors [85][99]. At least 5% of inhaled oxygen is converted to ROS, which naturally occurs as a byproduct of aerobic metabolism. In metabolic processes, cytochrome oxidase completely reduces most of the molecular oxygen to water in the mitochondria. Only partially reduced oxygen can react with long-chain molecules such as proteins, carbohydrates, lipids, and DNA. In higher organisms, RNS are produced by the oxidation of one of the terminal guanidonitrogen atoms of L-arginine [86][100] by nitric oxide synthase. NO can then be converted to various other forms of RNS [87][101]. The human body has a range of antioxidant defense mechanisms in place to protect against the potentially damaging effects of such active species., for example, by removing free radicals from the body. It is now known that oxidative stress, as well as ROS and RNS, negatively affect a number of cellular processes. When ROS exposure (or generation) increases or antioxidant levels fall, lipids, proteins, and DNA can be damaged, resulting in cell malfunction and even cell death [88][102]. Importantly, ROS participate in a number of physiological reactions of the body, such as the phagocytosis process [89][103]. Most importantly, the brain is particularly susceptible to oxidative stress because the level of aerobic respiration is high in the brain tissue. Additionally, brain tissue is rich in polyunsaturated fatty acids (PUFAs) that are susceptible to ROS damage [90][104]. A growing body of data indicates that ROS may also play an essential role in the pathophysiology of various neurological and psychiatric disorders, including mood disorders. Numerous studies have shown that individuals with neuropsychiatric disorders have higher levels of free radicals, lipid peroxides, pro-apoptotic markers, and altered antioxidant defense mechanisms [91][92][93][105,106,107]. A meta-analysis by Black et al. (2014) found oxidative stress to be elevated in people with MDD and/or depressive symptoms [94][108]. Importantly, oxidative stress is linked to various socio-demographic, health, and lifestyle variables, including socioeconomic status and smoking, which are also linked to depression [5][88][95][96][97][98][99][5,102,109,110,111,112,113]. Cigarette smoke has been demonstrated to decrease the levels of carotenoids and other antioxidants in human plasma [100][114]; it has been proposed that smoking may reduce carotenoid concentration by increasing metabolic rate, resulting in greater oxidative stress [101][115]. Today it is well known that antioxidants defend against the harmful effects of oxidative stress, which is believed to be associated with depression [102][103][116,117]. The antioxidant system consists of enzymatic antioxidants such as inter alia glutathione reductase, SOD, and catalase, as well as non-enzymatic forms such as vitamin C and E, N-acetylcysteine, reduced glutathione, flavonoids, and carotenoids. Carotenoids are natural antioxidants that can effectively prevent oxidative damage [67]. There is evidence that antioxidants exert a neuroprotective effect through their ability to repair the central nervous system and prevent oxidative stress-induced neurodegeneration. The total antioxidant capacity of a diet has been shown to have an inverse relationship with depression, anxiety, and stress [104][105][118,119]. Some data suggest that people with depression consume lower levels of antioxidants in the form of fruit and vegetables compared to those without [106][120]. In addition, data suggest that patients with depression have lower plasma vitamin E and C levels than those without [107][108][88,121]. Vitamin E has been found to exert an antidepressant-like effect in depressed animal models, and this has been attributed to it supporting the enzymatic glutathione-based antioxidant defense system in the hippocampus and prefrontal cortex [109][122]. However, growing evidence suggests that antioxidant treatment has proven unsatisfactory and even damaging in some oxidation-related diseases such as cancer [110][111][112][123,124,125]. While it iswe known that ROS plays a key role in defense against pathogens and intracellular signaling, the perception is that these compounds are harmful to cells. Likewise, antioxidants should not be regarded as purely beneficial agents. A clinical example of this is the finding that β-carotene supplementation in smokers leads to a significant increase in the incidence of lung cancer [113][114][126,127].5. Carotenoids and Their Role in the Course of Depression
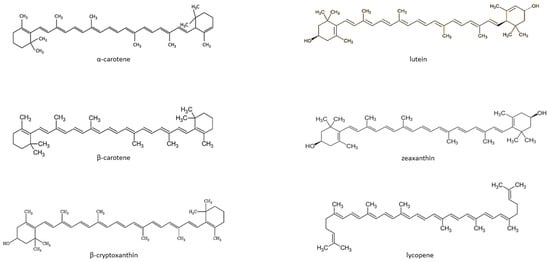
Carotenoids are fat-soluble color pigments that belong to the tetraterpene family, present in yellow-orange vegetables and fruits [115][128]. More than 700 carotenoids have been described, with the major forms being lycopene, β-carotene, ASTA, lutein, and zeaxanthin [116][117][118][129,130,131]. In nature, these pigments are found in many bacteria, fungi, and plants. The groups of carotenoids can also be divided into non-provitamin A and provitamin A (e.g., γ-carotene, β-carotene, α-carotene, and β-cryptoxanthin) [119][132]. The compounds can also be classified by the presence of specific functional groups: xanthophylls containing oxygen as a functional group (e.g., lutein, zeaxanthin), and carotenes containing only the parent hydrocarbon chain without any functional group (e.g., α-carotene, β-carotene, and lycopene) [119][132] (Figure 1).