Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by BEATRIZ CABALLERO GARCÍA.
There are several neurological diseases under which processes related to adult brain neurogenesis, such cell proliferation, neural differentiation and neuronal maturation, are affected. Melatonin can exert a relevant benefit for treating neurological disorders, given its well-known antioxidant and anti-inflammatory properties as well as its pro-survival effects. In addition, melatonin is able to modulate cell proliferation and neural differentiation processes in neural stem/progenitor cells while improving neuronal maturation of neural precursor cells and newly created postmitotic neurons.
- melatonin
- neural stem cells
- adult hippocampal neurogenesis
1. Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is a multifunctional hormone naturally produced and released rhythmically throughout the night by the pineal gland to regulate sleep–wake cycles [1]. The secretion of this neurohormone increases after the onset of darkness, reaching peak levels in the middle of the night, and gradually decreases in the second half of the night [2]. Light exposure stimulates the inhibition of melatonin production, and consequently, during the day its levels drop, becoming undetectable [3]. Furthermore, melatonin, once it is synthesized by the pineal gland, is promptly released into the bloodstream and is distributed among all tissues [4]. In particular, given the amphiphilic properties of melatonin, this neurohormone is able to cross biological barriers and enter cells, influencing tissue functions [5]. Additionally, melatonin is also locally synthetized in numerous cells and tissues, which presumably do not follow circadian rhythms.
Although melatonin is mainly referred to as the sleep hormone, this indolamine has been shown to exert antioxidant, anti-inflammatory and anti-apoptotic reprogramming in cellular homeostasis and disease (Figure 1). Melatonin mainly mediates its effects through MT1 and MT2 receptors, which belong to the superfamily of G protein-coupled receptors (GPCRs), by switching on/off intracellular signaling cascades. However, melatonin secretion, as well as the expression of melatonin receptors, has been widely proven to progressively decrease over the lifespan and in certain diseases [6,7][6][7], including neurodegenerative diseases or mental disorders [8]. This indicates that the downregulation of melatonin levels and their potential therapeutic effects may be involved in the onset and progression of diverse human diseases. Indeed, several recent studies endorse melatonin administration potentiality in various diseases, especially in neurodegenerative disorders [9,10][9][10].
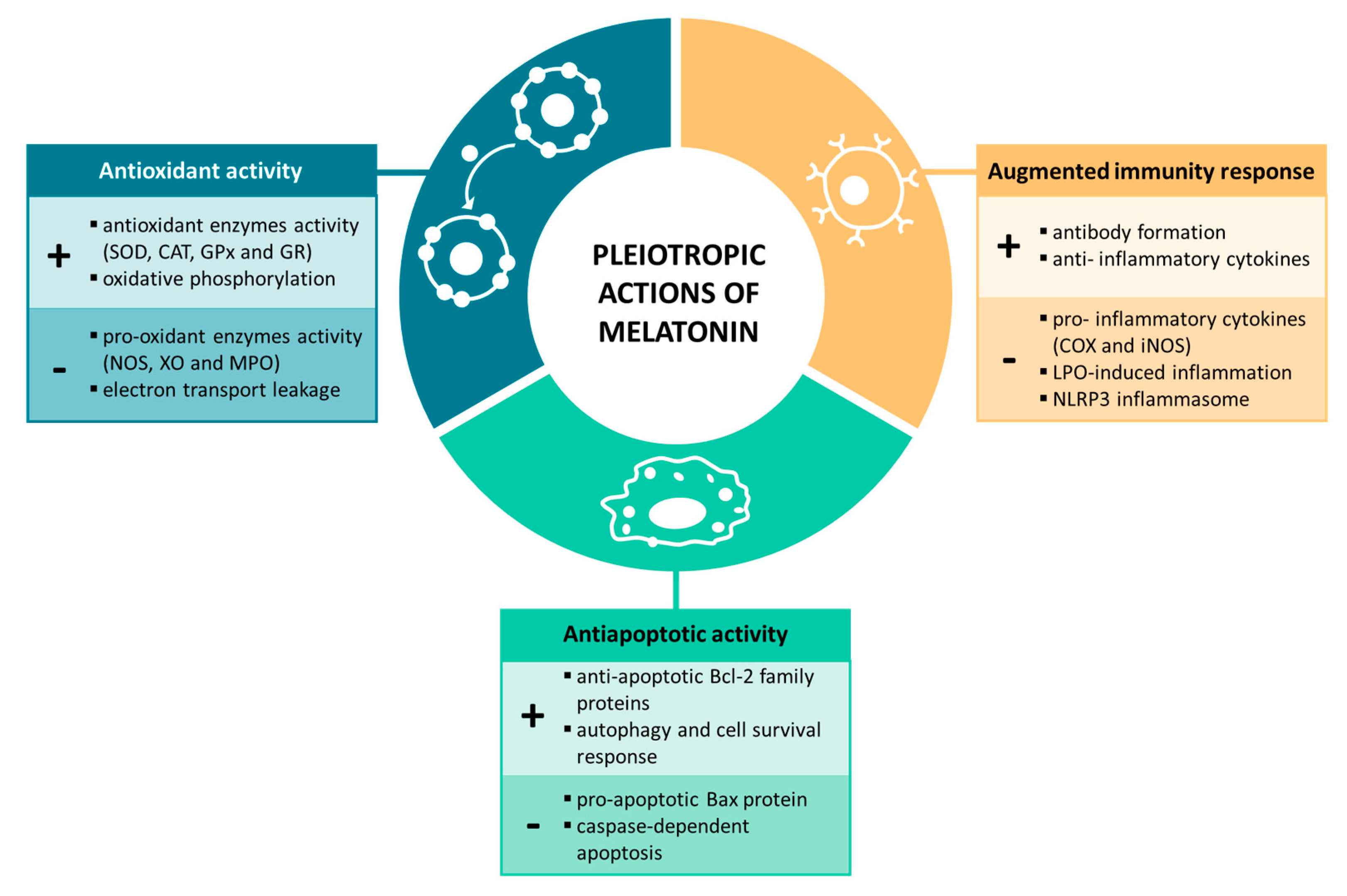
Figure 1. Melatonin’s pleiotropic actions on physiological processes.
2. Regulatory Role of Melatonin in Physiological Processes
2.1. Antioxidant Activity
Melatonin and its metabolic derivatives have been demonstrated to possess strong antioxidant properties against free radicals and are the reference agents in this field. This indolamine is considered an efficient scavenger of reactive oxygen species (ROS), reactive nitrogen species (RNS) and other oxidative agents [11]. Melatonin’s functions as an antioxidant include direct scavenging of free radicals, stimulation of the activity and efficiency of antioxidant enzymes, lowering the activation of pro-oxidant enzymes and improving the efficiency of mitochondrial respiration, thereby reducing ROS production [12]. First, melatonin, as an electron-rich molecule, acts as a potent endogenous free radical scavenger, forming stable end-products that are ultimately excreted by the organism. Additionally, this indolamine has been found to trigger the gene expression and activity of numerous antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR), among others [13,14][13][14]; it therefore contributes indirectly to the detoxification of free radicals. Furthermore, melatonin also protects against enzymes involved in the generation of free radicals. Abundant evidence indicates that melatonin inhibits nitric oxide synthase (NOS) activity, xanthine oxidase (XO) and myeloperoxidase (MPO) [15,16,17][15][16][17]. Finally, it should be noted that melatonin also acts within the mitochondrion, an organelle that is widely considered the major intracellular source of ROS production. In this context, the mechanisms by which melatonin protects mitochondria involve multiple pathways, from the increase in the activity of antioxidant enzymes while reducing pro-oxidant enzymes within the mitochondria to the stabilization of the mitochondrial inner membrane, the improvement of the oxidative phosphorylation and the reduction of electron transport leakage ROS production and the control of opening the mitochondrial permeability transition pore [12]. Thus, taking all these factors into account, melatonin not only contributes both directly and indirectly to the detoxification of free radicals, but it also avoids their production, favoring the maintenance of cellular homeostasis.
2.2. Immune System Properties
One of the most relevant pleiotropic effects of melatonin is the modulation of the immune system, reducing chronic and acute inflammation [18,19][18][19]. This relationship is mainly established through the bidirectional communication between the pineal gland, as a neuroendocrine interface, and the immune system. As early as 1926, Berman described for the first time this potential interrelation, evidenced after kittens were fed pineal glands from bullocks, and observed increased learning, activity and resistance against infectious diseases. After this finding, numerous investigations focused on better understanding this relationship. In particular, to discover this tight connection, two experimental approaches have mainly been addressed: (a) pinealectomy and (b) the synchronization between rhythmic melatonin synthesis and the immune response [20,21][20][21]. Largely, pinealectomy causes a reduction in the size of both primary and secondary lymphoid organs, ultimately affecting innate responses [22]. Moreover, the circadian rhythm for the release of melatonin also influences antibody formation. When melatonin levels are higher, there is greater stimulation of antibody formation, thus contributing to an augmented immune response [23,24][23][24].
Generally, basic and clinical research suggests that the anti-inflammatory effects of melatonin are mediated by the modulation of anti- and proinflammatory cytokines [25,26][25][26]. Melatonin was reported to inhibit the production of two of the main inflammatory mediators, cyclooxygenase (COX) and inducible NOS (iNOS), by modulating nuclear factor kappa B (NF-κB) translocation [27,28,29,30][27][28][29][30]. In addition, this indolamine has also been shown to alleviate inflammasome activation. Melatonin was demonstrated to reduce lipopolysaccharide (LPS)-induced inflammation and the formation of the NLRP3 inflammasome in mouse adipocytes, thus inhibiting caspase-1 and IL-1 activation and the NLRP3 inflammasome-mediated pyroptosis [31,32][31][32]. Given that inflammation is implicated in the development and progression of several diseases, such as neurological disorders, the immunomodulatory effect of melatonin has gained increasing attention in recent years.
2.3. Anti-apoptotic Activity
Melatonin is considered a master regulator of cell death via the inhibition of apoptotic responses and the activation of survival pathways. Mitochondria are one of the main cellular organelles that sense and respond to many stressors, leading to adaptive and maladaptive responses through the regulation of diverse signaling pathways, among which it is worth highlighting apoptosis and autophagy [33]. Under adverse conditions, mitochondrial function is affected, which ultimately triggers the release of cytochrome c (CytC) and apoptosis-inducing factor (AIF) into the cytosol, and therefore, the activation of apoptosis machinery. Caspases are also critical regulatory molecules that generate a cascade of signaling events, controlling cell death in disease. It has been found that melatonin is capable repressing the mitochondrial-mediated apoptotic response by enhancing a compensatory pathway. This neurohormone increases the expression of the anti-apoptotic Bcl-2 family proteins, but it inhibits the activity of the pro-apoptotic Bax protein by acting on the SIRT1/NF-κB axis [34,35][34][35]. Additionally, melatonin also suppresses caspase-dependent apoptosis [36]. This indolamine was found to silence the caspase-1 pathway and reduce the overexpression and activation of caspase-3 [37,38][37][38]. Furthermore, melatonin not only suppresses apoptotic responses but also stimulates the Akt pathway, mediating the activation of autophagy and its roles in cell survival [10,39][10][39]. Therefore, melatonin has been widely demonstrated to be a potent anti-apoptotic agent mainly due to its regulatory action on proteins involved in mitochondria-mediated apoptosis and on cell survival mechanisms.
3. Neurogenesis in the Adult Brain
The current dogma “that new neurons can and do form in the adult mammalian brain” is well accepted [40]. In the adult brain, neurogenesis is a process that starts with cell proliferation and ends with new functional neurons that integrate into existing neural circuits. There are two “canonical” regions of the mammalian adult brain that generate new neurons: (a) the border of the lateral ventricles of the brain (subventricular zone) and (b) the subgranular zone of the hippocampal dentate gyrus [41,42,43,44][41][42][43][44]. Several non-canonical regions also contain neural progenitor cells, including the neocortex, striatum and hypothalamus [44].
In adult hippocampal neurogenesis, the differentiation of adult neural stem cells into mature functional neurons proceeds through a clearly defined set of cellular stages starting from (a) type-1 cells (radial glia-like cells) that express different markers such as glial fibrillar acidic protein (GFAP), nestin, SRY-box transcription factor 2 (SOX2) and brain lipid-binding protein (BLBP) to (b) type-2a and 2b cells (transiently amplifying progenitor cells) that are positive for specific markers (e.g., nestin, SOX2, achaete-scute family bHLH transcription factor 1 (ASCL1), T-box transcriptional factor 2 (TBR-2), etc.); and (c) type-3 cells (neuroblasts), which undergo migration and final maturation to functional neurons and express specific markers such as polysialylated neural cell adhesion molecule (PSA-NCAM) and neurogenic differentiation factor 1 (NeuroD1) [41,42,44][41][42][44]. The newly created neurons developing from neural stem cells in the hippocampus integrate into pre-existing neural networks of the granular neuron layer of the dentate gyrus to participate in learning and memory processes [43,44,45][43][44][45].
In addition to ciliated ependymal cells, the subventricular zone of lateral ventricles presents a varied niche of neural stem cells and precursors, including (a) proliferating neuroblasts (type A cells) that express different neuronal markers (Tubulin beta 3 Class III (TUBB3) and NeuroD1) and are able to migrate to the olfactory bulb via the rostral migratory pathway (b) slowly proliferating cells (type B cells), which express multipotent neural stem cell markers (nestin and GFAP) and show capacity for self-renewal and differentiation toward neurons and glial cells; and (c) transiently amplifying progenitors (type C cells) that show nestin expression and a very active state of proliferation [41].
Neurogenesis alteration can be a consequence of a decrease in the pool of neural stem cells, alterations in the molecular microenvironment that do not favor cell proliferation and/or cell differentiation or because neural stem cells and progenitor cells cannot respond to neurogenic signals in the aged brain and/or under neurodegeneration [42,46][42][46]. Therefore, interventions that can maintain adult brain neurogenesis are key to improving neurological functions, even in the late phases of aging, and especially under conditions of neurodegeneration [42,43,45,46][42][43][45][46].
Melatonin promotes neuroprotection due to its antioxidant, anti-inflammatory and anti-apoptotic properties [10,47,48,49,50][10][47][48][49][50]. Melatonin also plays an important role in the regulation of neurogenesis [51,52,53][51][52][53]. Therefore, melatonin is a potential treatment for neurodegenerative and neurologic disorders associated with an impairment of neurogenesis, including normal brain aging, dementia, stress, depression, stroke, traumatic brain injury, etc. [53,54,55][53][54][55]. The first evidence of the neurogenic potential of melatonin comes from studies in pinealectomized rats, which showed that an important reduction in melatonin levels also leads to decreases in adult hippocampal neurogenesis [56]. Exogenous administration of melatonin in these animals restored their melatonin levels and the functionality of neurogenesis [56]. Similarly, chronic administration of luzindole (an antagonist for melatonin receptors) also demonstrated that a lack of melatonin significantly affects hippocampal neurogenesis in C57BL/6 adult mice [57].
Melatonin is able to modulate several processes involved in adult brain neurogenesis including survival, proliferation and neuronal differentiation processes of neural stem/progenitor cells, normal migration of neuronal precursors and survival and maturation of newly created neurons (formation and growth of dendrites, complexity of dendrite trees, length of axonal prolongations, processes of synaptic plasticity, etc.) [42,46,51,55,58,59,60,61,62][42][46][51][55][58][59][60][61][62]. These neurogenic actions of melatonin may involve several signaling pathways and molecular mechanisms that impact adult brain neurogenesis [53[53][55][62][63],55,62,63], as summarized in Figure 2.
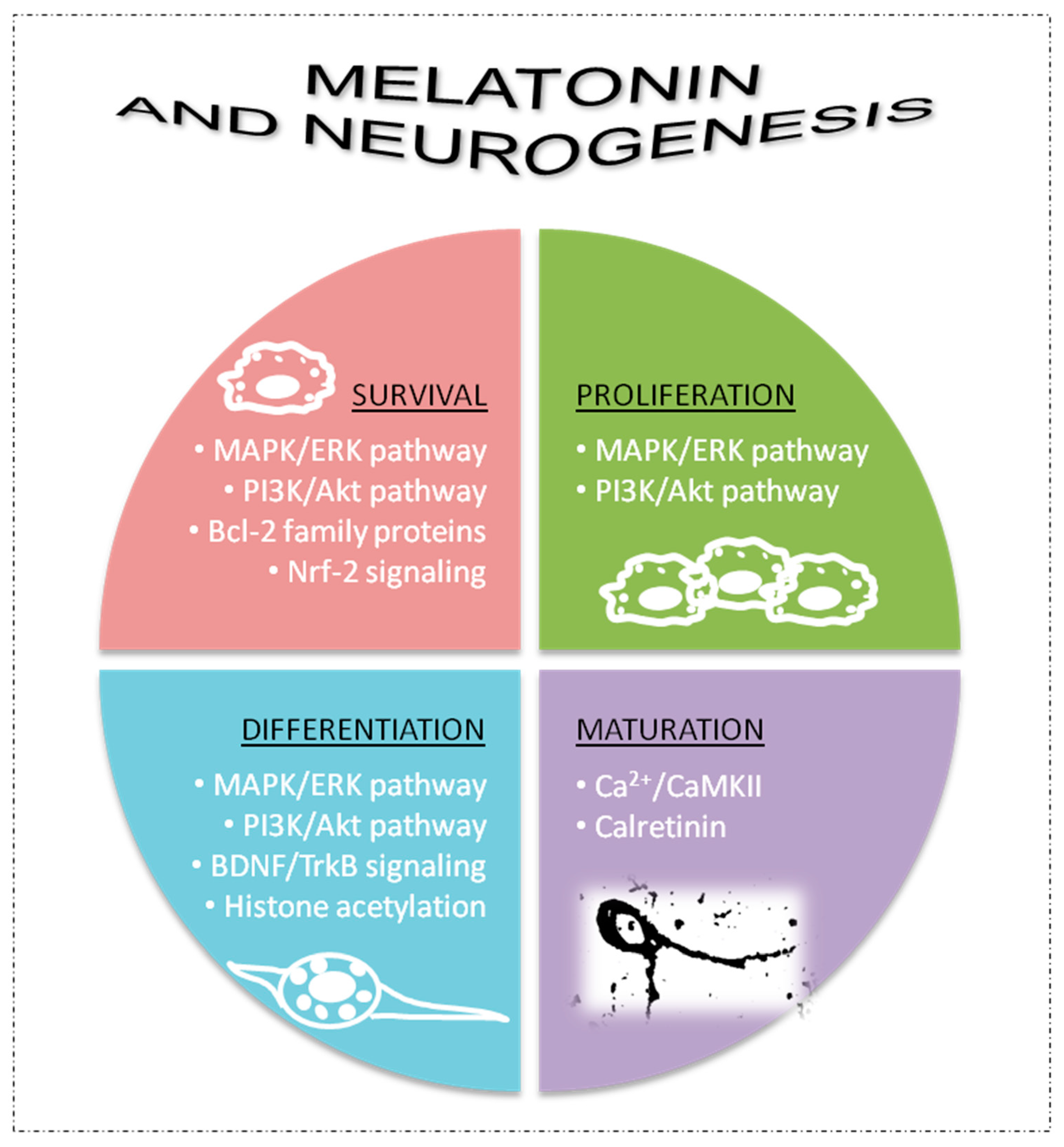
Figure 2. Some of mechanisms involved in the neurogenic potential of melatonin. AkT, protein kinase B; Bcl-2, B-cell lymphoma; BDNF, brain-derived neurotrophic factor; Ca2+/CaMKII, calcium/calmodulin-dependent kinase II; ERK, extracellular signal-regulated kinases; GDNF, glial cell line-derived neurotrophic factor; Nrf-2, nuclear factor erythroid 2-related factor 2; MAPK, mitogen-activated protein kinases; PI3K, phosphoinositide 3-kinase; TrkB, tropomycin-receptor kinase B.
Neurogenic pathways of melatonin can be mediated by its membrane receptors (MT1/2) [51,57][51][57]. Signaling pathways related to activation of mitogen-activated protein kinases (MAPK) and extracellular signal-regulated kinases 1/2 (ERK 1/2) as well as phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt) are frequently involved in melatonin-receptor-related effects on cell survival, proliferation and neuronal differentiation of neural stem/progenitor cells [51,52,53][51][52][53]. However, other neurogenic actions of melatonin can be independent of its receptors, including melatonin’s capacity to promote cell survival of neural progenitor and precursor cells through regulation of apoptosis by modulating Bcl-2 family proteins [51[51][53],53], and due to its antioxidant properties by modulation of Nrf2 signaling [51,53][51][53]. Increases in neurotrophic factors, such as brain-derived neurotrophic factor (BDNG), are also involved in the capacity of melatonin to activate neuronal differentiation processes in neural stem/progenitor cells independently of its receptors [51,53][51][53]. Histone acetylation is also promoted by melatonin to activate neuronal differentiation, which may or may not involve its membrane receptors [51]. Melatonin can activate tropomycin-receptor kinase B (TrkB) signaling via the MT1 receptor or by increasing BDNF levels to impact cell survival and proliferation [51,52,53][51][52][53]. Finally, melatonin also promotes maturation processes in new postmitotic neurons, including the formation, growth and complexity of dendrites and synaptic plasticity [59,61][59][61]. In this last case, melatonin is able to directly interact with intracellular proteins, such as calcium/calmodulin-dependent kinase II (Ca2+/CaMKII) and the Ca2+ binding protein calretinin, to modulate the cytoskeleton and promote different neuronal maturation processes [64,65,66][64][65][66] (Figure 2).
These beneficial effects of melatonin on different parameters of neurogenesis (survival, proliferation, differentiation, maturation, etc.) have been demonstrated by using different concentrations of melatonin (from nM to μM) during acute treatments (from hours to days) in several models in vitro, including commercial cell lines (e.g., rat PC12 and mouse C17.2 cell lines), primary cultures of neural stem/progenitor cells obtained from the brain or spinal cord of adult rats and mice, cultured mesenchymal stem cells from human amniotic fluid, induced pluripotent stem cells from mice and adult rat/mouse hippocampus organotypic cultures [55]. Likewise, several animal models have confirmed the neurogenic potential of melatonin in both acute and chronic melatonin treatments [55]. For instance, various studies in Balb/C mice have demonstrated positive effects of melatonin promoting the proliferation and survival of neural progenitor cells as well as the survival, maturation and complexity of dendrites in the new postmitotic immature neurons in the dentate gyrus of the hippocampus of these adult mice [54,67,68][54][67][68]. Melatonin also modulates the structural plasticity of axons in granule cells in the dentate gyrus of Balb/C mice by regulating mossy fiber projections to establish new functional synapses in the hippocampus [61]. Similarly, melatonin also increased the survival of neural progenitor cells and postmitotic immature neurons in the dentate gyrus of adult C57BL/6 mice [58]. More recent studies have shown that melatonin restores the functionality of adult hippocampal neurogenesis during the accelerated and pathological brain aging of SAMP8 mice [46]. The benefits of melatonin acquire an especial relevance in the success of treatments of different nervous tissue lesions by transplants with mesenchymal stem cells. Antioxidant and anti-inflammatory properties of melatonin may improve the survival and functionality of transplanted mesenchymal stem cells. This then leads to favorable outcomes in different experimental treatments, for instance, in focal cerebral ischemia and neurodegenerative processes of Alzheimer’s disease [62]. Neurogenic differentiation from mesenchymal stem cells specifically requires wingless-integration-1 (Wnt) expression and activation of c-Jun N-terminal kinases (JNK) pathway [62]. Finally, melatonin is also able to revert those alterations on neurogenesis that are induced by several drugs or toxic compounds including lipopolysaccharides, valproic acid, methamphetamines, cuprizone, methotrexate, dexamethasone, metformin, scopolamine, 5-fluorouracil and corticosteroids [53,55,62,69,70,71,72,73,74][53][55][62][69][70][71][72][73][74].
References
- Srinivasan, V.; Spence, W.D.; Pandi-Perumal, S.R.; Zakharia, R.; Bhatnagar, K.P.; Brzezinski, A. Melatonin and human reproduction: Shedding light on the darkness hormone. Gynecol. Endocrinol. 2009, 25, 779–785.
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180.
- Menendez-Pelaez, A.; Howes, K.A.; Gonzalez-Brito, A.; Reiter, R.J. N-acetyltransferase activity, hydroxyindole-O-methyltransferase activity, and melatonin levels in the Harderian glands of the female Syrian hamster: Changes during the light:dark cycle and the effect of 6-parachlorophenylalanine administration. Biochem. Biophys. Res. Commun. 1987, 145, 1231–1238.
- Cheung, R.T.; Tipoe, G.L.; Tam, S.; Ma, E.S.; Zou, L.Y.; Chan, P.S. Preclinical evaluation of pharmacokinetics and safety of melatonin in propylene glycol for intravenous administration. J. Pineal Res. 2006, 41, 337–343.
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochimica Biophysica Acta 1999, 1472, 206–214.
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729.
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326.
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158.
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480.
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534.
- Bañón-Arnao, M.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168.
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146.
- Esparza, J.L.; Gomez, M.; Rosa Nogues, M.; Paternain, J.L.; Mallol, J.; Domingo, J.L. Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J. Pineal Res. 2005, 39, 129–136.
- Gomez, M.; Esparza, J.L.; Nogues, M.R.; Giralt, M.; Cabre, M.; Domingo, J.L. Pro-oxidant activity of aluminum in the rat hippocampus: Gene expression of antioxidant enzymes after melatonin administration. Free Radic. Biol. Med. 2005, 38, 104–111.
- Bettahi, I.; Pozo, D.; Osuna, C.; Reiter, R.J.; Acuna-Castroviejo, D.; Guerrero, J.M. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J. Pineal Res. 1996, 20, 205–210.
- Leon, J.; Escames, G.; Rodriguez, M.I.; Lopez, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrion, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033.
- Teixeira, A.; Morfim, M.P.; de Cordova, C.A.; Charao, C.C.; de Lima, V.R.; Creczynski-Pasa, T.B. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J. Pineal Res. 2003, 35, 262–268.
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017, 62, e12389.
- Dong, Y.; Fan, C.; Hu, W.; Jiang, S.; Ma, Z.; Yan, X.; Deng, C.; Di, S.; Xin, Z.; Wu, G.; et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016, 60, 253–262.
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200.
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683.
- McKinney, T.D.; Vaughan, M.K.; Reiter, R.J. Pineal influence on intermale aggression in adult house mice. Physiol. Behav. 1975, 15, 213–216.
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin. Exp. Immunol. 1987, 68, 384–391.
- Carrillo-Vico, A.; Reiter, R.J.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 2006, 7, 423–431.
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525.
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14.
- Tocharus, J.; Chongthammakun, S.; Govitrapong, P. Melatonin inhibits amphetamine-induced nitric oxide synthase mRNA overexpression in microglial cell lines. Neurosci. Lett. 2008, 439, 134–137.
- Tamura, E.K.; Cecon, E.; Monteiro, A.W.; Silva, C.L.; Markus, R.P. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J. Pineal Res. 2009, 46, 268–274.
- Rahim, I.; Djerdjouri, B.; Sayed, R.K.; Fernandez-Ortiz, M.; Fernandez-Gil, B.; Hidalgo-Gutierrez, A.; Lopez, L.C.; Escames, G.; Reiter, R.J.; Acuna-Castroviejo, D. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. 2017, 63, e12410.
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3863–3875.
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414.
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436.
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017, 102, 203–216.
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357.
- Sun, F.Y.; Lin, X.; Mao, L.Z.; Ge, W.H.; Zhang, L.M.; Huang, Y.L.; Gu, J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 2002, 33, 48–56.
- Shi, L.; Liang, F.; Zheng, J.; Zhou, K.; Chen, S.; Yu, J.; Zhang, J. Melatonin Regulates Apoptosis and Autophagy Via ROS-MST1 Pathway in Subarachnoid Hemorrhage. Front. Mol. Neurosci. 2018, 11, 93.
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 55, 26–35.
- Jalili, S.; Ehsanpour, A.A.; Javadirad, S.M. The role of melatonin on caspase-3-like activity and expression of the genes involved in programmed cell death (PCD) induced by in vitro salt stress in alfalfa (Medicago sativa L.) roots. Bot. Stud. 2022, 63, 19.
- Li, Y.; Guo, Y.; Fan, Y.; Tian, H.; Li, K.; Mei, X. Melatonin Enhances Autophagy and Reduces Apoptosis to Promote Locomotor Recovery in Spinal Cord Injury via the PI3K/AKT/mTOR Signaling Pathway. Neurochem. Res. 2019, 44, 2007–2019.
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73.
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed Res. Int. 2015, 2015, 727542.
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 2018, 38, 10401–10410.
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells 2019, 8, 125.
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020, 49, 3–16.
- Moreno-Jimenez, E.P.; Terreros-Roncal, J.; Flor-Garcia, M.; Rabano, A.; Llorens-Martin, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553.
- Cachan-Vega, C.; Vega-Naredo, I.; Potes, Y.; Bermejo-Millo, J.C.; Rubio-Gonzalez, A.; Garcia-Gonzalez, C.; Antuna, E.; Bermudez, M.; Gutierrez-Rodriguez, J.; Boga, J.A.; et al. Chronic Treatment with Melatonin Improves Hippocampal Neurogenesis in the Aged Brain and Under Neurodegeneration. Molecules 2022, 27, 5543.
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernandez, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Gutierrez-Cuesta, J.; Pallas, M.; Camins, A.; et al. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in senescence-accelerated mice. J. Pineal Res. 2008, 45, 302–311.
- Gutierrez-Cuesta, J.; Sureda, F.X.; Romeu, M.; Canudas, A.M.; Caballero, B.; Coto-Montes, A.; Camins, A.; Pallas, M. Chronic administration of melatonin reduces cerebral injury biomarkers in SAMP8. J. Pineal Res. 2007, 42, 394–402.
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernandez, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Pallas, M.; Camins, A.; Rodriguez-Colunga, M.J.; et al. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J. Pineal Res. 2009, 46, 106–114.
- Gutierrez-Cuesta, J.; Tajes, M.; Jimenez, A.; Camins, A.; Pallas, M. Effects of melatonin in the brain of the senescence-accelerated mice-prone 8 (SAMP8) model. Rev. Neurol. 2011, 52, 618–622.
- Chu, J.; Tu, Y.; Chen, J.; Tan, D.; Liu, X.; Pi, R. Effects of melatonin and its analogues on neural stem cells. Mol. Cell. Endocrinol. 2016, 420, 169–179.
- Yu, X.; Li, Z.; Zheng, H.; Ho, J.; Chan, M.T.; Wu, W.K. Protective roles of melatonin in central nervous system diseases by regulation of neural stem cells. Cell Prolif. 2017, 50, e12323.
- Romero, A.; Morales-Garcia, J.A.; Ramos, E. Melatonin: A multitasking indoleamine to modulate hippocampal neurogenesis. Neural Regen. Res. 2023, 18, 503–505.
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Benitez-King, G.; Castro-Garcia, M.; Ortiz-Lopez, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58.
- Leung, J.W.; Cheung, K.K.; Ngai, S.P.; Tsang, H.W.; Lau, B.W. Protective Effects of Melatonin on Neurogenesis Impairment in Neurological Disorders and Its Relevant Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5645.
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009, 47, 313–317.
- Ortiz-Lopez, L.; Perez-Beltran, C.; Ramirez-Rodriguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221.
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191.
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37.
- Ramirez-Rodriguez, G.; Ocana-Fernandez, M.A.; Vega-Rivera, N.M.; Torres-Perez, O.M.; Gomez-Sanchez, A.; Estrada-Camarena, E.; Ortiz-Lopez, L. Environmental enrichment induces neuroplastic changes in middle age female Balb/c mice and increases the hippocampal levels of BDNF, p-Akt and p-MAPK1/2. Neuroscience 2014, 260, 158–170.
- Ramirez-Rodriguez, G.B.; Olvera-Hernandez, S.; Vega-Rivera, N.M.; Ortiz-Lopez, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2018, 20, 73.
- Hardeland, R. Melatonin and the Programming of Stem Cells. Int. J. Mol. Sci. 2022, 23, 1971.
- Wang, J.; Xia, Z.; Sheng, P.; Rui, Y.; Cao, J.; Zhang, J.; Gao, M.; Wang, L.; Yu, D.; Yan, B.C. Targeting MicroRNA-144/451-AKT-GSK3beta Axis Affects the Proliferation and Differentiation of Radial Glial Cells in the Mouse Hippocampal Dentate Gyrus. ACS Chem. Neurosci. 2022, 13, 897–909.
- Ramirez-Rodriguez, G.; Gomez-Sanchez, A.; Ortiz-Lopez, L. Melatonin maintains calcium-binding calretinin-positive neurons in the dentate gyrus during aging of Balb/C mice. Exp. Gerontol. 2014, 60, 147–152.
- Dominguez-Alonso, A.; Valdes-Tovar, M.; Solis-Chagoyan, H.; Benitez-King, G. Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: Participation of the Ca++/Calmodulin complex. Int. J. Mol. Sci. 2015, 16, 1907–1927.
- Argueta, J.; Solis-Chagoyan, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velazquez-Moctezuma, J.; Benitez-King, G. Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity. Int. J. Mol. Sci. 2022, 23, 2479.
- Ramirez-Rodriguez, G.B.; Palacios-Cabriales, D.M.; Ortiz-Lopez, L.; Estrada-Camarena, E.M.; Vega-Rivera, N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal- and Ventral- Dentate Gyrus Concomitantly with Its Antidepressant-Like Effect in Male Balb/C Mice. Int. J. Mol. Sci. 2020, 21, 1724.
- Vega-Rivera, N.M.; Ortiz-Lopez, L.; Granados-Juarez, A.; Estrada-Camarena, E.M.; Ramirez-Rodriguez, G.B. Melatonin Reverses the Depression-associated Behaviour and Regulates Microglia, Fractalkine Expression and Neurogenesis in Adult Mice Exposed to Chronic Mild Stress. Neuroscience 2020, 440, 316–336.
- Aranarochana, A.; Chaisawang, P.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Protective effects of melatonin against valproic acid-induced memory impairments and reductions in adult rat hippocampal neurogenesis. Neuroscience 2019, 406, 580–593.
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H.; et al. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, e01388.
- Sirichoat, A.; Krutsri, S.; Suwannakot, K.; Aranarochana, A.; Chaisawang, P.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin protects against methotrexate-induced memory deficit and hippocampal neurogenesis impairment in a rat model. Biochem. Pharm. 2019, 163, 225–233.
- Sirichoat, A.; Suwannakot, K.; Chaisawang, P.; Pannangrong, W.; Aranarochana, A.; Wigmore, P.; Welbat, J.U. Melatonin attenuates 5-fluorouracil-induced spatial memory and hippocampal neurogenesis impairment in adult rats. Life Sci. 2020, 248, 117468.
- Suwannakot, K.; Sritawan, N.; Prajit, R.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Protects against the Side-Effects of 5-Fluorouracil on Hippocampal Neurogenesis and Ameliorates Antioxidant Activity in an Adult Rat Hippocampus and Prefrontal Cortex. Antioxidants 2021, 10, 615.
- Suwannakot, K.; Sritawan, N.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Attenuates Methotrexate-Induced Reduction of Antioxidant Activity Related to Decreases of Neurogenesis in Adult Rat Hippocampus and Prefrontal Cortex. Oxidative Med. Cell. Longev. 2022, 2022, 1596362.
More
