Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that plays a crucial role in cells adapting to a low-oxygen environment by facilitating a switch from oxygen-dependent ATP production to glycolysis. Mediated by membrane type-1 matrix metalloproteinase (MT1-MMP) expression, Munc-18-1 interacting protein 3 (Mint3) binds to the factor inhibiting HIF-1 (FIH-1) and inhibits its suppressive effect, leading to HIF-1α activation. Defects in Mint3 generally lead to improved acute inflammation, which is regulated by HIF-1α and subsequent glycolysis, as well as the suppression of the proliferation and metastasis of cancer cells directly through its expression in cancer cells and indirectly through its expression in macrophages or fibroblasts associated with cancer. Mint3 in inflammatory monocytes enhances the chemotaxis into metastatic sites and the production of vascular endothelial growth factors, which leads to the expression of E-selectin at the metastatic sites and the extravasation of cancer cells. Fibroblasts express L1 cell adhesion molecules in a Mint3-dependent manner and enhance integrin-mediated cancer progression. In pancreatic cancer cells, Mint3 directly promotes cancer progression. Naphthofluorescein, a Mint3 inhibitor, can disrupt the interaction between FIH-1 and Mint3 and potently suppress Mint3-mediated inflammation, cancer progression, and metastasis without causing marked adverse effects.
- Mint3
- hypoxia
- HIF-1
1. Introduction
2. HIF Proteins
As a key factor associated with the adaptation of cells to low-oxygen environments, HIFs are known to play crucial roles as transcription factors [12,13][8][9]. HIF proteins include three alpha subunits (HIF-1α, HIF-2α, and HIF-3α) and one beta subunit (HIF-1β); they are heterodimers composed of one of the three alpha subunits and HIF-1β. HIF alpha subunits are stably expressed under hypoxic conditions, whereas HIF-1β is stably expressed independent of oxygen [14][10]. All HIF proteins share a basic domain and a dimerization domain within the N-terminal. However, alpha subunits but not beta subunits possess an N-terminal transactivation domain (NAD) in the C-terminal, and only HIF-1α and HIF-2α have the C-terminal transactivation domain (CAD) [15,16][11][12]. These structural differences among the four types of HIF subunits lead to differences in protein stability and transcriptional activity regulation. The HIF subunits also show variations in their expression locus and target genes. While HIF-1α is ubiquitously expressed in human tissues and upregulates the expression of glycolysis-related genes, HIF-2α is expressed in specific tissues, such as tumor vascular cells and macrophages, and promotes erythropoietin and iron metabolism [17,18,19][13][14][15]. HIF-3α has an important role in adipogenesis, and its gene expression is regulated by HIF-2α activity [20][16]. The stability of all alpha subunits is conditionally regulated by oxygen concentration.3. Mechanisms of Mint3-Mediated HIF-1 Activation
3.1. Mint3 Indirectly Upregulates HIF-1α Activity through Its Interaction with FIH-1
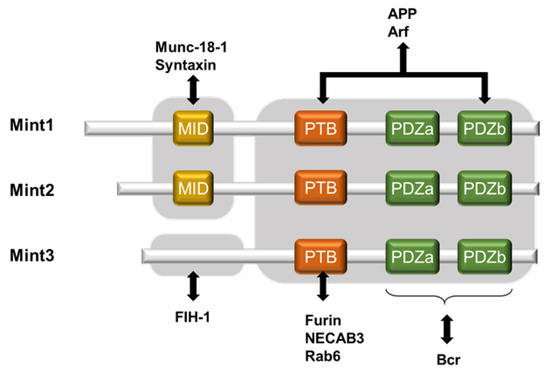
Mint3, also known as amyloid-beta A4 precursor protein-binding family A member 3, is a novel protein that positively regulates HIF-1α [30][17]. The Mint family consists of three isoforms: Mint1, Mint2, and Mint3 (also known as APBA1, APBA2, and APBA3; X11, X11-like, and X11-like2; and X11α, β, and γ, respectively). Mint1 and Mint2 bind with Munc-18-1, which is involved in neural homeostasis [31][18]. Mint3 shows ubiquitous expression in various tissues, with the lowest level in the testis, according to Okamoto and Sudhof [32][19]. Its intracellular localization is mainly at the Golgi apparatus where it interacts with furin [33][20]. Mint1-3 were originally identified as neural proteins that interact with the YENPTY motif within amyloid precursor proteins (APPs), which are conserved in the cytoplasmic region of APP and modulate their activities [32,34,35][19][21][22]. All three Mint isoforms share highly conserved structures in their C-terminal region, which comprise one phosphotyrosine-binding (PTB) domain followed by tandem PDZ domains (PDZa and PDZb). Through these two domains, Mint3 functions as an adaptor protein. With its PTB domain, Mint3 binds to proteins such as furin, N-terminal EF-hand calcium binding protein 3 (NECAB3), and Rab6 [32,33,36,37][19][20][23][24]. However, only Mint1 and Mint2 retain the N-terminal region, which contains Munc-18-1 interacting domain; because Mint-3 lacks the domain, it is unable to interact with Munc-18-1 [32][19] (Figure 1). Additionally, a recent structural analysis of Mint3 revealed that its N-terminal region is intrinsically disordered [40][25]. Thus, the sequences of the N-terminal region of Mint3 differ substantially from those of the other Mint proteins, and, as a result, Mint3 has divergent binding partners at its N-terminal region.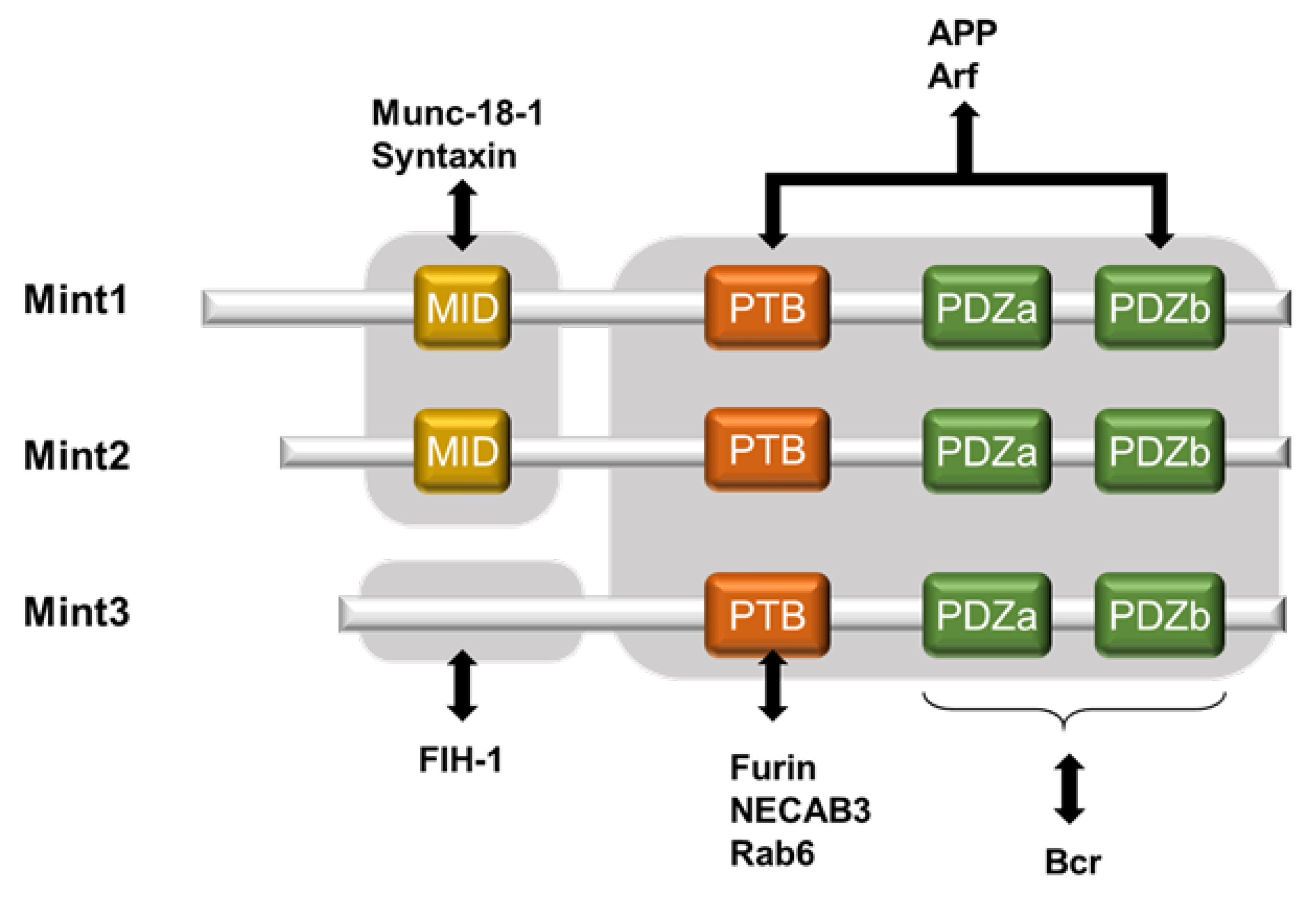
3.2. Factors That Support Mint3-Mediated HIF-1α Activation
4. Mint3 Mediates Inflammatory Responses
Macrophages are the key players in innate immunity and rely on constant HIF-1α-mediated glycolysis regardless of oxygen conditions [4[4][34],50], which is dependent on Mint3 activity. Upon stimulation or infection by various pathogens, macrophages are involved in producing cytokines and chemokines, growth factors, and reactive oxygen species (ROS) during inflammation to protect the host. However, the overactivation of the immune system can rather be toxic due to septic shock [51][35]. Stimulation by lipopolysaccharides (LPS), which are a toxic component of the outer membrane of Gram-negative bacteria, causes septic shock. This toxicity is a consequence of a cytokine storm mediated by the glycolysis-dependent secretion of cytokines associated with increased motility and invasiveness of macrophages, which involve the Mint3–FIH-1–HIF-1α axis [52][36]. Macrophages gain “lethal force” by Mint3 expression under LPS-driven immune reaction. In an immune response to influenza virus (IFV), Mint3 depletion efficiently improves influenza pneumonia by alleviating the production of cytokines and chemokines in macrophages (but not in dendritic cells) through two mechanisms: the upregulation of Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) α activity and the stabilization of IκB [53][37]. AMPK is activated through its phosphorylation on Adenosine 5’-triphosphate (ATP) starvation [54][38] and downregulates nuclear factor kappa B (NF-κB) activity [55][39]. The depletion of Mint3 downregulates glycolysis-mediated ATP production and activates AMPK and the subsequent inhibition of NF-κB. Concurrently, Mint3 deficiency leads to the glycolysis-independent stabilization of IκB, which causes the simultaneous inhibition of NF-κB. Pyroptosis is a mechanism of programmed cell death that is associated with innate immunity and occurs when cells encounter intracellular bacterial infection. It was initially pointed out by Friedlander and was named by D’Souza to distinguish it from apoptosis [57,58][40][41]. Canonical pyroptosis signals involve the formation of inflammasomes, which mostly comprise Nod-like receptors (NLRs)/absent in melanoma 2 (AIM2); apoptosis-associated speck-like protein (ASC) as an adaptor protein; and the inflammatory caspase-1, whereas caspase-11/4/5 could serve as an inflammatory caspase in non-canonical pyroptosis. Inflammasomes process the pyroptosis-executing protein gasdermin D (GSDMD) and interleukin (IL)-1β/IL-18. Listeria monocytogenes (LM) is a common foodborne pathogen that causes zoonotic diseases in Western countries by infecting various types of cells. Upon innate infection by LM, the host operates a protective mechanism by clearing out the bacteria through the production of ROS and nitric oxide (NO) induced by the activation of pyroptosis [59,60,61,62][42][43][44][45].5. Role of Mint3 in Cancer Progression
The effects of Mint3 on the innate immune system may be applicable in cancers because Mint3 has the potential to increase cancer malignancy and metastatic features through its expression in inflammatory monocytes (IMs), which are defined with the following markers: Gr-1/Ly6C+, CD11b/CD115+ [72][46]. In addition, a relationship between Mint3 activity in cancer cells and cancer-associated fibroblasts (CAFs) has been previously reported [11,73,74][47][48][49]. Because Mint3 has key roles in enhancing cancer progression and metastasis by upregulating HIF-1α, which is also related to the tumor microenvironments, it could be an attractive target for cancer treatment, as tumor-microenvironments-related factors are gaining more attraction, which is described as one of the important hallmarks of cancers [75][50].
5.1. Impact of Mint3 Activity in Cancer Cells
Solid tumors in human bodies are often exposed to low-oxygen environments due to the disadvantage in physical distance from the blood vessels and obtaining sufficient oxygen. In order to adapt to such circumstances, cancer cells tend to retain high HIF-1α activities and gain malignancies. Although the depletion of Mint3 in the host of xenograft models has no effects on tumor progression, its depletion in cancer cells results in HIF-1α suppression, which is associated with downregulated glycolysis, angiogenesis, and anti-proliferative effects. These Mint3-related features are commonly seen in the xenografts of various types of cancer cells (e.g., breast cancer, MDA-MB-231; fibrosarcoma, HT-1080; epidermoid carcinoma, A431; non-small cell lung cancer, A549; and urothelial carcinoma, RT-112) [11,73,74,76][47][48][49][51].5.2. Metastatic Ability of Cancer Cells Achieved through Mint3 Expression in IM
Using the PyMT breast cancer mouse model, which develops palpable breast cancers that metastasize to the lung, Qian et al. reported that IMs, which are characterized by Gr-1/Ly6C+, CD11b/CD115+ markers, play a key role in the lung metastasis of breast cancer in a C–C motif chemokine ligand 2 (CCL2)-dependent manner [81][52]. Other studies using xenograft mouse models of various types of cancers have shown that Mint3 plays an important role in this mechanism of metastasis [72,82][46][53]. Cancer cells and the surrounding stromal cells are the sources of CCL2, and they increase the number of IMs in the peripheral blood. Both individual depletion of Mint3 or HIF-1α in IMs leads to the decreased production of vascular endothelial growth factor (VEGF) to comparable levels. These effects of Mint3 or HIF-1α depletion on VEGF expression are consistent with the fact that VEGF is one of the target genes of HIF-1α [72,83][46][54].5.3. Supportive Effect of Mint3 Expression in CAFs on Cancer Progression
CAFs are one of the factors that have a crucial role in promoting cancer malignancy through ECM remodeling, immune crosstalk, and metabolic effects [84][55]. These features of CAFs are achieved by functioning as a source of various secretory proteins, such as growth factors, cytokines, and exosomes, whereas the adherent molecules in CAFs could also increase tumor malignancy in a Mint3-dependent mechanism. Mint3 is also involved in the proliferation of cancer cells by regulating the expression of L1 cell adhesion molecule (L1CAM) in fibroblasts. L1CAM is a cell adhesion molecule that acts as a binding partner of heterodimeric integrins, such as α5β1, αvβ3, αIIbβ3, and αvβ5 [85[56][57],86], and triggers downstream signaling pathways, such as the MAPK and PI3K/Akt pathways [85,87][56][58]. Fibroblasts express L1CAM in a Mint3-dependent manner, which enables CAFs to promote direct contact with cancer cells expressing integrin α5β1. Therefore, Mint3 also plays a role in enhancing the proliferation of cancer cells through CAFs [11][47].6. Therapeutic Efficacy of Targeting Mint3-Related Environments
In some cases, the activity of HIF-1α is toxic in terms of its ability to promote inflammatory diseases or cancer malignancies. Thus, HIF-1α has long been regarded as a beneficial therapeutic target. Many efforts have been made to develop HIF-1α inhibitors, but none have been successful, although some clinical trials are still underway. This is probably due to the ubiquitous expression of HIF-1α, which influences various factors. The first HIF-2α inhibitor, belzutifan (Welireg), was clinically approved for patients with VHL diseases and some types of cancers in 2021. Naphthofluorescein (Naph) was first identified as a potent Mint3 inhibitor. It effectively disrupts the protein interaction between Mint3 and FIH-1, leading to the suppression of HIF-1α activity with reduced expression of HIF-1α-targeting genes, glycolysis, and ATP production [76][51]. The inhibitory effect of Naph on Mint3 in inflammatory diseases effectively diminishes cytokine synthesis; upon LPS stimulation, this is well reflected in the increased survival of mice with LPS-induced inflammatory diseases. Moreover, Naph retains its inhibitory potential against chemotaxis of IMs toward CCL2–expressing cancer cells and the surrounding stromal cells, thereby reducing the expression of E-selectin in metastatic lung cancers without marked adverse effects. Notably, the pharmacological effect of Naph on metastasis is not affected in Mint3-deficient mice, which implies that Naph can suppress metastasis specifically by inhibiting Mint3 [76][51].References
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314.
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344.
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218.
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Forster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003, 112, 645–657.
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998, 12, 149–162.
- Rezvani, H.R.; Ali, N.; Serrano-Sanchez, M.; Dubus, P.; Varon, C.; Ged, C.; Pain, C.; Cario-Andre, M.; Seneschal, J.; Taieb, A.; et al. Loss of epidermal hypoxia-inducible factor-1alpha accelerates epidermal aging and affects re-epithelialization in human and mouse. J. Cell Sci. 2011, 124, 4172–4183.
- Takubo, K.; Goda, N.; Yamada, W.; Iriuchishima, H.; Ikeda, E.; Kubota, Y.; Shima, H.; Johnson, R.S.; Hirao, A.; Suematsu, M.; et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010, 7, 391–402.
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237.
- Wang, G.L.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 1993, 90, 4304–4308.
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996, 271, C1172–C1180.
- Pugh, C.W.; O’Rourke, J.F.; Nagao, M.; Gleadle, J.M.; Ratcliffe, P.J. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J. Biol. Chem. 1997, 272, 11205–11214.
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82.
- Rankin, E.B.; Biju, M.P.; Liu, Q.; Unger, T.L.; Rha, J.; Johnson, R.S.; Simon, M.C.; Keith, B.; Haase, V.H. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Investig. 2007, 117, 1068–1077.
- Renassia, C.; Peyssonnaux, C. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol. 2019, 26, 125–130.
- Wang, V.; Davis, D.A.; Haque, M.; Huang, L.E.; Yarchoan, R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005, 65, 3299–3306.
- Hatanaka, M.; Shimba, S.; Sakaue, M.; Kondo, Y.; Kagechika, H.; Kokame, K.; Miyata, T.; Hara, S. Hypoxia-inducible factor-3alpha functions as an accelerator of 3T3-L1 adipose differentiation. Biol. Pharm. Bull. 2009, 32, 1166–1172.
- Sakamoto, T.; Seiki, M. Mint3 enhances the activity of hypoxia-inducible factor-1 (HIF-1) in macrophages by suppressing the activity of factor inhibiting HIF-1. J. Biol. Chem. 2009, 284, 30350–30359.
- Okamoto, M.; Sudhof, T.C. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J. Biol. Chem. 1997, 272, 31459–31464.
- Okamoto, M.; Südhof, T.C. Mint 3: A ubiquitous mint isoform that does not bind to munc18-1 or -2. Eur. J. Cell Biol. 1998, 77, 161–165.
- Han, J.; Wang, Y.; Wang, S.; Chi, C. Interaction of Mint3 with Furin regulates the localization of Furin in the trans-Golgi network. J. Cell Sci. 2008, 121, 2217–2223.
- Borg, J.P.; Ooi, J.; Levy, E.; Margolis, B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell Biol. 1996, 16, 6229–6241.
- Tanahashi, H.; Tabira, T. X11L2, a new member of the X11 protein family, interacts with Alzheimer’s beta-amyloid precursor protein. Biochem. Biophys. Res. Commun. 1999, 255, 663–667.
- Teber, I.; Nagano, F.; Kremerskothen, J.; Bilbilis, K.; Goud, B.; Barnekow, A. Rab6 interacts with the mint3 adaptor protein. Biol. Chem. 2005, 386, 671–677.
- Nakaoka, H.J.; Hara, T.; Yoshino, S.; Kanamori, A.; Matsui, Y.; Shimamura, T.; Sato, H.; Murakami, Y.; Seiki, M.; Sakamoto, T. NECAB3 Promotes Activation of Hypoxia-inducible factor-1 during Normoxia and Enhances Tumourigenicity of Cancer Cells. Sci. Rep. 2016, 6, 22784.
- Ten, T.; Nagatoishi, S.; Maeda, R.; Hoshino, M.; Nakayama, Y.; Seiki, M.; Sakamoto, T.; Tsumoto, K. Structural and thermodynamical insights into the binding and inhibition of FIH-1 by the N-terminal disordered region of Mint3. J. Biol. Chem. 2021, 297, 101304.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67.
- Sakamoto, T.; Seiki, M. Integrated functions of membrane-type 1 matrix metalloproteinase in regulating cancer malignancy: Beyond a proteinase. Cancer Sci. 2017, 108, 1095–1100.
- Yana, I.; Weiss, S.J. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell 2000, 11, 2387–2401.
- Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370, 61–65.
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451.
- Barbolina, M.V.; Stack, M.S. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin. Cell Dev. Biol. 2008, 19, 24–33.
- Sakamoto, T.; Seiki, M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells 2009, 14, 617–626.
- Sakamoto, T.; Seiki, M. A membrane protease regulates energy production in macrophages by activating hypoxia-inducible factor-1 via a non-proteolytic mechanism. J. Biol. Chem. 2010, 285, 29951–29964.
- Michl, J.; Ohlbaum, D.J.; Silverstein, S.C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J. Exp. Med. 1976, 144, 1465–1483.
- Riedemann, N.C.; Guo, R.F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524.
- Hara, T.; Mimura, K.; Abe, T.; Shioi, G.; Seiki, M.; Sakamoto, T. Deletion of the Mint3/Apba3 gene in mice abrogates macrophage functions and increases resistance to lipopolysaccharide-induced septic shock. J. Biol. Chem. 2011, 286, 32542–32551.
- Uematsu, T.; Fujita, T.; Nakaoka, H.J.; Hara, T.; Kobayashi, N.; Murakami, Y.; Seiki, M.; Sakamoto, T. Mint3/Apba3 depletion ameliorates severe murine influenza pneumonia and macrophage cytokine production in response to the influenza virus. Sci. Rep. 2016, 6, 37815.
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262.
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. (Berl.) 2011, 89, 667–676.
- Friedlander, A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986, 261, 7123–7126.
- D’Souza, C.A.; Heitman, J. Dismantling the Cryptococcus coat. Trends Microbiol. 2001, 9, 112–113.
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2014, 26, 209–220.
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082.
- Boockvar, K.S.; Granger, D.L.; Poston, R.M.; Maybodi, M.; Washington, M.K.; Hibbs, J.B., Jr.; Kurlander, R.L. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 1994, 62, 1089–1100.
- Uematsu, T.; Tsuchiya, K.; Kobayashi, N.; Seiki, M.; Inoue, J.I.; Kaneko, S.; Sakamoto, T. Mint3 depletion-mediated glycolytic and oxidative alterations promote pyroptosis and prevent the spread of Listeria monocytogenes infection in macrophages. Cell Death Dis. 2021, 12, 404.
- Hara, T.; Nakaoka, H.J.; Hayashi, T.; Mimura, K.; Hoshino, D.; Inoue, M.; Nagamura, F.; Murakami, Y.; Seiki, M.; Sakamoto, T. Control of metastatic niche formation by targeting APBA3/Mint3 in inflammatory monocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E4416–E4424.
- Nakaoka, H.J.; Tanei, Z.; Hara, T.; Weng, J.S.; Kanamori, A.; Hayashi, T.; Sato, H.; Orimo, A.; Otsuji, K.; Tada, K.; et al. Mint3-mediated L1CAM expression in fibroblasts promotes cancer cell proliferation via integrin alpha5beta1 and tumour growth. Oncogenesis 2017, 6, e334.
- Kanamori, A.; Matsubara, D.; Saitoh, Y.; Fukui, Y.; Gotoh, N.; Kaneko, S.; Seiki, M.; Murakami, Y.; Inoue, J.I.; Sakamoto, T. Mint3 depletion restricts tumor malignancy of pancreatic cancer cells by decreasing SKP2 expression via HIF-1. Oncogene 2020, 39, 6218–6230.
- Ikeda, J.; Ohe, C.; Tanaka, N.; Yoshida, T.; Saito, R.; Atsumi, N.; Kobayashi, T.; Kinoshita, H.; Tsuta, K.; Sakamoto, T. Hypoxia inducible factor-1 activator munc-18-interacting protein 3 promotes tumour progression in urothelial carcinoma. Clin. Transl. Discov. 2023, 3, e158.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Sakamoto, T.; Fukui, Y.; Kondoh, Y.; Honda, K.; Shimizu, T.; Hara, T.; Hayashi, T.; Saitoh, Y.; Murakami, Y.; Inoue, J.I.; et al. Pharmacological inhibition of Mint3 attenuates tumour growth, metastasis, and endotoxic shock. Commun. Biol. 2021, 4, 1165.
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225.
- Hara, T.; Murakami, Y.; Seiki, M.; Sakamoto, T. Mint3 in bone marrow-derived cells promotes lung metastasis in breast cancer model mice. Biochem. Biophys. Res. Commun. 2017, 490, 688–692.
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- Kiefel, H.; Bondong, S.; Hazin, J.; Ridinger, J.; Schirmer, U.; Riedle, S.; Altevogt, P. L1CAM: A major driver for tumor cell invasion and motility. Cell Adhes. Migr. 2012, 6, 374–384.
- Kiefel, H.; Pfeifer, M.; Bondong, S.; Hazin, J.; Altevogt, P. Linking L1CAM-mediated signaling to NF-kappaB activation. Trends Mol. Med. 2011, 17, 178–187.
- Chen, D.L.; Zeng, Z.L.; Yang, J.; Ren, C.; Wang, D.S.; Wu, W.J.; Xu, R.H. L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J. Hematol. Oncol. 2013, 6, 43.
