Epilepsy is a neurological disorder characterized by hypersynchronous recurrent neuronal activities and seizures, as well as loss of muscular control and sometimes awareness. Clinically, seizures have been reported to display daily variations. Conversely, circadian misalignment and circadian clock gene variants contribute to epileptic pathogenesis. Elucidation of the genetic bases of epilepsy is of great importance because the genetic variability of the patients affects the efficacies of antiepileptic drugs (AEDs).
- epilepsy
- epileptic genes
- circadian clock
1. Introduction
2. Circadian Rhythms in Human Epilepsies
As early as 1885, William R. Gowers observed three groups of epilepsy patients in daily patterns: diurnal, nocturnal, and diffuse [12][7]. Diurnal seizures occur at certain times of the day, whereas nocturnal seizures tend to occur primarily at bedtime and at night [110][20]. Recently, SeizureTracker (Springfield, VA, USA) and NeuroVista (Melbourne, VIC, Australia) were employed to analyze the rhythmic patterns of seizures and found that the seizure rates of approximately 80% of 1118 patients displayed daily variations [111][21]. The mechanisms underpinning why seizures display daily variation are not clear. A legitimate hypothesis is that the circadian clock contributes to seizure rhythmicity. However, little is known about the circadian roles in epilepsy and seizures. The International League Against Epilepsy (ILAE) broadly categorizes seizures into focal seizures, generalized seizures, and seizures of unknown onset [112,113,114][22][23][24]. Focal seizures occur only in discrete brain regions limited to one hemisphere, generalized seizures involve large bilateral brain areas, even the whole brain cortex, and seizures of unknown onset do not belong to the focal or generalized categories [112,114,115][22][24][25]. EEG recordings of generalized seizures have been revealed to be significantly more robust in the morning than in the afternoon [116][26]. Furthermore, focal seizures have been shown to manifest a predictable daily pattern [16][11]. Parietal lobe epilepsy (PLE) occurs primarily around the end of sleep in the morning [15,20][10][15]. Two peaks of PLE were found in Hofstra’s study of 450 times of seizures: one peaking around 05:00 to 11:00, and the other peaking around 17:00–23:00 [117][27]. In contrast, occipital and temporal lobe epilepsy often occurs in the afternoon [18,20,118][13][15][28]. Temporal lobe epilepsy has been classified into mesial (MTLE), lesional (LTLE), and neocortical temporal lobe (NTLE) epilepsy. MTLE displays two peaks, 07:00–08:00 and 16:00–17:00 [20,117,119[15][27][29][30],120], respectively, whereas LTLE peaks around 11:00, and NTLE peaks around 11:00–17:00 [117][27] and early morning [120][30]. On the other hand, interictal epileptiform discharges (IEDs), sharp waves in the EEG background between seizures, show a nocturnal predominance and often occur during NREM sleep [16,121,122][11][31][32]. However, recent studies have shown that the peak of nocturnal predominance in interictal epileptiform activity (IEA) was independent of the region of seizure irritability, monitored by an implantable brain stimulator (RNS® system, Neuropace, Mountain View, CA, USA) continuously for a more extended period [120,123][30][33]. Together, these studies indicated that the circadian rhythm of seizures was robust and endogenous, independent of antiseizure dosing [111[21][34],124], indicating a possible circadian role in epileptic pathogenesis.3. The Circadian Clock
Circadian rhythms, as biological rhythms with a period of approximately 24 h, are regulated and controlled by an endogenous time-keeping mechanism, i.e., the circadian clock [128,129,130,131][35][36][37][38]. In mammals, the central clock is situated at the suprachiasmatic nuclei (SCN) of the anterior hypothalamus [132][39]. The mammalian SCN neurons exhibit higher activity in electrical physiology and metabolism during the daytime [133,134][40][41]. External light is received by intrinsically photosensitive retinal ganglion cells (ipRGCs) and transmitted to the SCN via the retinohypothalamic tract (RHT) [135][42]. The efferent from the SCN projects to the pineal gland and drives the rhythmic release of melatonin, a sleep-promoting hormone. Since light is known to inhibit melatonin synthesis, the daily oscillation of melatonin is similar in both diurnal and nocturnal animals [136,137][43][44] and helps to synchronize peripheral organs in the body [138][45]. Intriguingly, most organs, tissues, and cells display circadian rhythmicity, regulated by the local peripheral clock, as well as neural, hormonal, and metabolic cues from the SCN [139][46]. Three transcription-translation feedback loops as molecular time-keeping mechanisms are known to generate, regulate, and maintain circadian rhythms [140,141][47][48]. In the primary loop, the CLOCK: BMAL1 heterodimer as the positive limb activates the expression of target genes, including Per genes (Per1, Per2, and Per3 ) and Cry genes (Cry1 and Cry2) via binding to E-box (5′-CACGTG-3′) and E’-box (5′-CACGTT-3′) in their promoter regions [142][49], whereas the PER: CRY heterodimer as the negative limb interferes with the transcriptional activity of the CLOCK-BMAL1 heterodimer and turns off their expression [143][50]. In the second loop, Rorα/β and Rev-erbα/β are regulated by CLOCK and BMAL1 via E-box, whereas their proteins RORα/β and REV-ERBα/β activate and suppress Bmal1 by competing for binding to the RORE (retinoic-acid-related orphan receptor response element) in the Bmal1 promoter region (Figure 31). In the third loop, D-box-containing proline and acidic amino-acid-rich basic leucine zipper (PAR bZip) genes Dbp (albumin D-box-binding protein), Hlf (hepatic leukemia factor), Tef (thyrotroph embryonic factor), and E4bp4/Nfil3 (E4 promoter-binding protein 4/nuclear factor interleukin-3-regulated protein/nuclear factor, interleukin 3 regulated) are all regulated by CLOCK and BMAL1 via E-box, whereas their proteins DBP, HIF, TEF, and E4BP4/NFIL3 bind to D-box in the promoter regions of their target genes, where DBP, HIF, and TEF activate D-box-containing genes and E4BP4/NFIL3 represses them [144][51]. Among these three circadian-clock-controlled cis-elements-mediated transcriptional feedback loops, the E/E’-box-mediated loop plays the dominant role in the circadian clock [145][52]. However, the E/E’-box-mediated loop, combined with the RORE-mediated loop and the D-box-mediated loop, forms the necessary transcriptional repression and delays for oscillating approximately 24 h a day. In particular, E/E’-box, D-box, and RORE act in the morning, evening, and night, respectively [146,147][53][54]. In addition, the mechanistic/mammalian target of the rapamycin (mTOR) pathway, implicated in numerous neurological disorders, has been shown to contribute to circadian regulation [148,149][55][56] by activating circadian clock genes through the phosphorylation of the translation factor S6K1 [148,150,151][55][57][58]. Specifically, S6K1 phosphorylates GSK3β, which, in turn, phosphorylates CLOCK, BMAL1, and REV-ERB [152][59].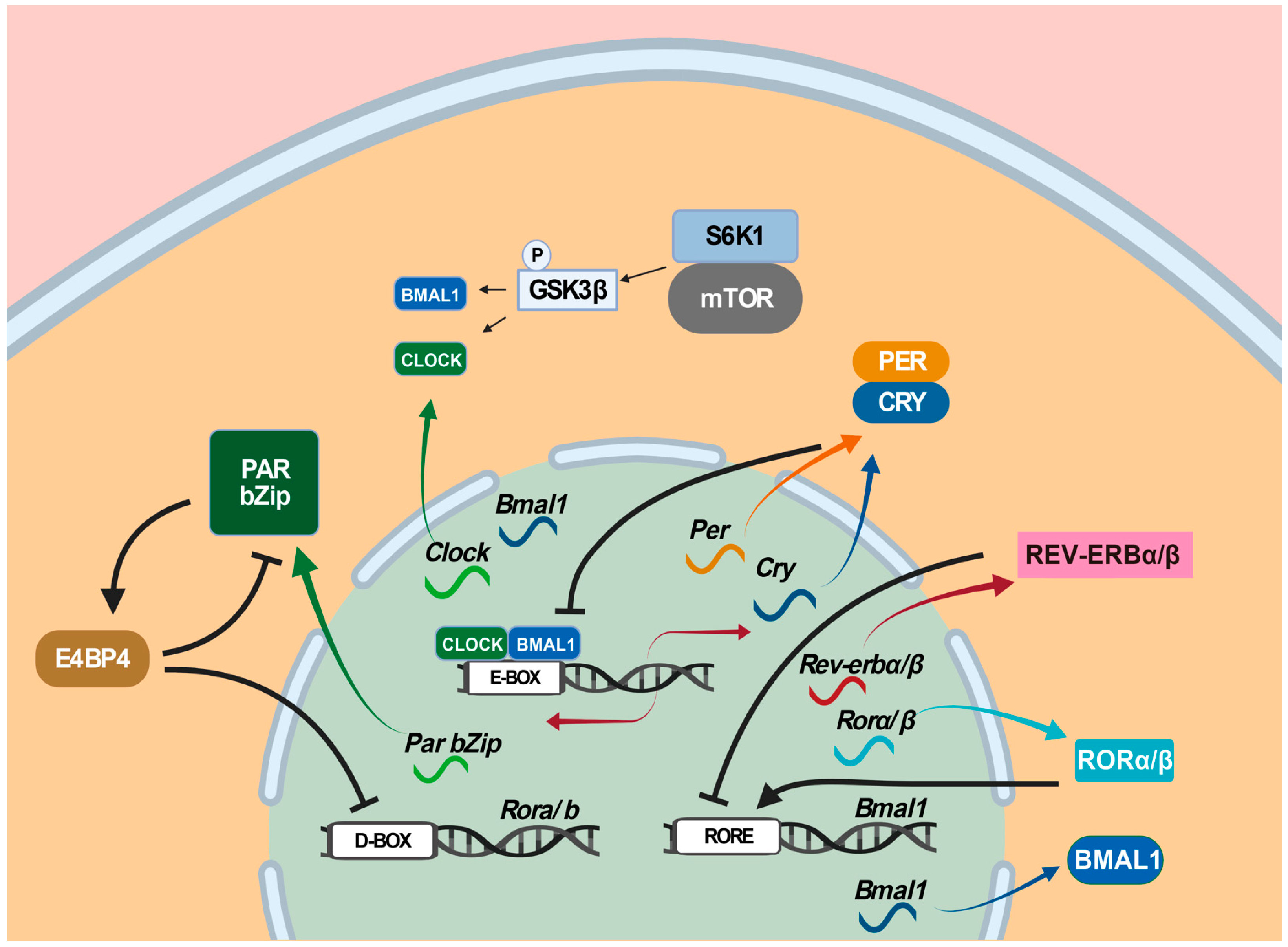
4. The Roles of Circadian Clock Genes in Epilepsies
5. Mutual Effects between Epilepsy and Sleep
Sleep and epilepsy are reciprocally affected. On the one hand, NREM sleep, especially NREM stage 1 (N1) and stage 2 (N2) sleep, facilitates epileptogenesis, while REM sleep inhibits it [156][63]. Specifically, REM sleep has the most suppressive effect during the EEG desynchronization period [157][64], whereas NREM sleep facilitates seizures due to the effect of the synchronous discharge of the thalamocortical network [158[65][66],159], as evidenced by the fact that 95% of seizures occur in NREM sleep [160][67]. An interesting study demonstrated that a small lesion in focal cortical dysplasia (FCD) type II patients is highly associated with sleep-related epilepsy [161][68]. Interictal epileptiform discharges (IEDs) have been used to evaluate seizure exploding [162][69]. Melatonin, a sleep-promoting hormone, appears to contribute to the nocturnal predominance of IEDs during sleep [163][70], and reduced melatonin levels with shifted phases were reported in epilepsy patients [126,164,165][71][72][73]. In adult patients, frontal lobe epilepsy is the archetypal sleep-related epilepsy that tends to occur during sleep, whereas juvenile myoclonic epilepsy often occurs in the morning [166][74].
On the other hand, epileptic activity has been shown to affect sleep continuity, which increases waking time after sleep onset, reduces REM sleep quality, and delays the first REM sleep episode in epilepsy patients [169][75]. Epilepsy patients often suffer from severe sleep disturbances such as excessive daytime sleepiness, sleep fragmentation, and insomnia [170][76]. Nocturnal seizures may lead to severe sleep fragmentation and even NREM parasomnia [171][77], and diurnal seizures also result in the alteration of the sleep architecture [172][78]. The epileptic activity also alters the sleep oscillations, likely through desynchronizing hippocampal IED and remote cortical spindles [173][79]. The sleep architecture of JME patients is severely altered with prolonged REM onset latency and decreased REM percentage [174][80].
Hence, sleep problems are prevalent among epilepsy patients, and reciprocal interactions between epilepsy and sleep should be underscored in epilepsy treatment. Various questionnaire-based instruments, such as the Pittsburgh Sleep Quality Index (PSQI) and the Sleep Condition Indicator, should be employed to evaluate the sleep status of patients, and possible comorbid sleep disorders also must be assessed. In particular, comorbid sleep disorders, once verified, should be treated separately. Generally, after epileptic patients are cured with drug treatment or surgeries, their sleep quality is expected to be improved with normal sleep patterns and melatonin levels [166][74]. Good quality of sleep helps contain seizures.
6. A Chronomodulated Strategy for Epilepsy Therapy
6.1. Circadian Mechanisms Underlying Epileptogenesis
Although our understanding of the mechanisms underlying epilepsy remains limited, mounting evidence indicates circadian involvement in epilepsy pathogenesis, as numerous types of human epilepsies display robust daily rhythmicity [111][21]. Future efforts will aim to identify circadian biomarkers by elucidating molecular genetic mechanisms underlying how the circadian clock regulates the robust rhythmicity of these epilepsies. In doing so, the circadian clock system and possible circadian-clock-regulated epilepsy processes such as the hypothalamus–pituitary–adrenal (HPA) axis and the hypothalamus–pituitary–gonadal (HPG) axis should be investigated. The circadian clock regulates the HPA axis [273,274][81][82] and the HPG axis [275[83][84],276], which are known to contribute to epilepsy pathogenesis [277,278,279][85][86][87]. In some epilepsies, it would be worthwhile to investigate how the circadian clock acts through the HPA axis or the HPG axis to regulate epilepsy pathogenesis. Furthermore, it would be intriguing to determine whether the core circadian clock genes and circadian-clock-controlled epilepsy genes harbor mutations or whether the normal rhythmic expression patterns of these circadian clock genes and circadian-clock-controlled epilepsy genes are altered in individual patients. This line of investigation should provide insights into the circadian regulation of the dynamics of the pathogenesis of a particular epilepsy, which should provide cues for the time-of-day delivery of AEDs (Figure 52).
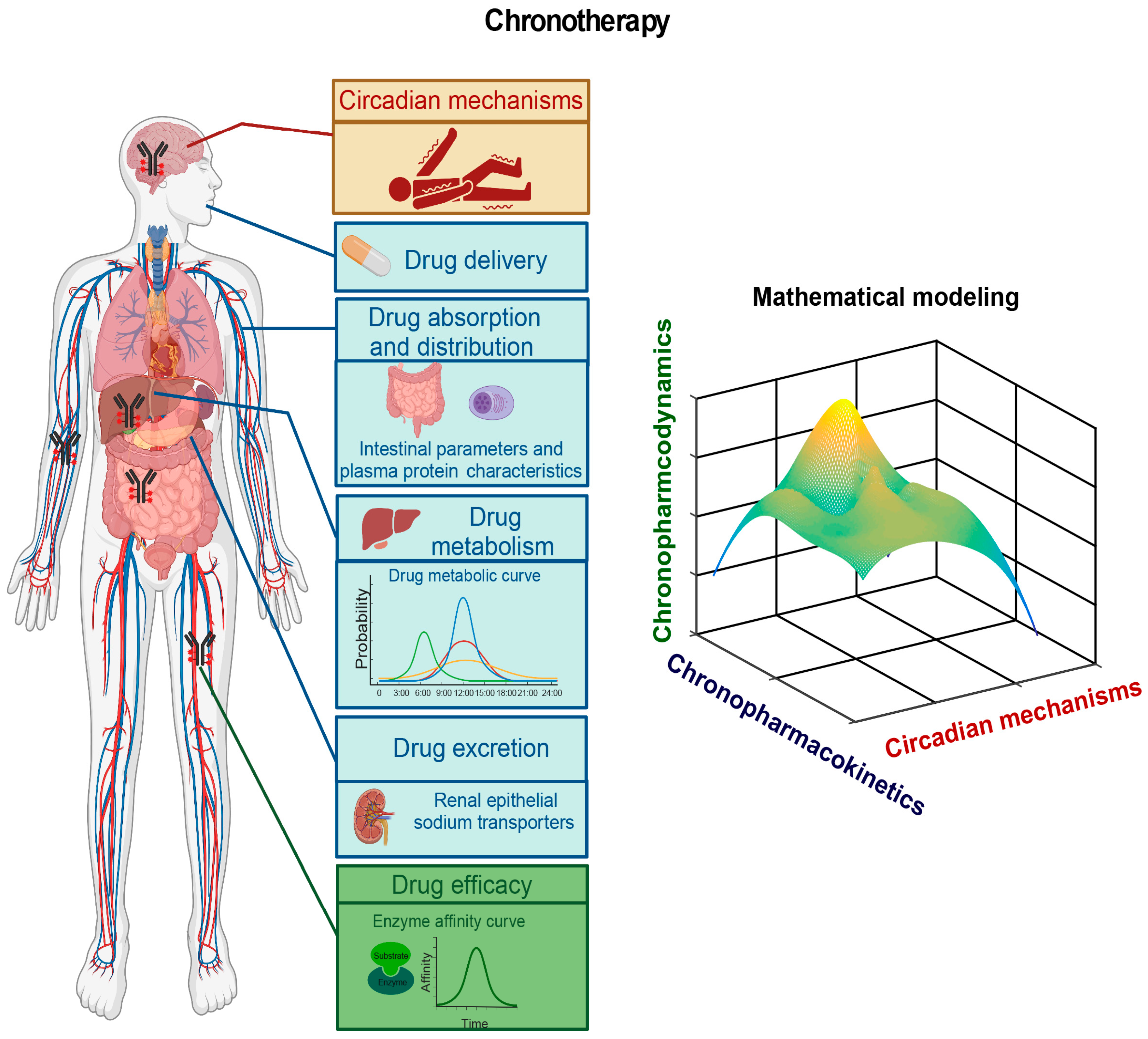
6.2. Pharmacokinetic and Pharmacodynamic Studies of AEDs
6.3. Epileptic Chronotherapy
In a study with a small cohort of 17 children with nocturnal or early-morning seizures, instead of conventional administration of equal doses of AEDs in the morning and evening each day, two times the morning AED dose was delivered in the evening with the equivalent total dosage. After the 5-month differential dosing treatment, 64.7% (11/17) of patients became seizure-free, and 88.2% (15/17) experienced a ≥50% reduction in seizures [24][19]. AED therapy with CBZ treatment was shown to significantly reduce urinary melatonin metabolite levels of epileptic patients during 06:00–14:00 and 22:00–06:00 [126][71]. Further, a 5–10 mg evening melatonin delivery can effectively reduce the frequency of epileptic attacks [297][94].References
- Leonardi, M.; Ustun, T.B. The global burden of epilepsy. Epilepsia 2002, 43, 21–25.
- Kwan, P.; Brodie, M.J. Effectiveness of First Antiepileptic Drug. Epilepsia 2001, 42, 1255–1260.
- Schmidt, D.; Löscher, W. Drug Resistance in Epilepsy: Putative Neurobiologic and Clinical Mechanisms. Epilepsia 2005, 46, 858–877.
- Semah, F.; Picot, M.C.; Adam, C.; Broglin, D.; Arzimanoglou, A.; Bazin, B.; Cavalcanti, D.; Baulac, M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 1998, 51, 1256–1262.
- Vogel, F. Moderne Probleme der Humangenetik; Springer: Berlin/Heidelberg, Germany, 1959; pp. 52–125.
- Langdon-Down, M.; Brain, W.R. Time of day in relation to convulsions in epilepsy. Lancet 1929, 213, 1029–1032.
- Gowers, W.R. Epilepsy and Other Chronic Convulsive Diseases: Their Causes, Symptoms & Treatment; William Wood & Company: West Chester, PA, USA, 1885.
- Fisher, R.S.; Boas, W.V.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472.
- Horne, J.A.; Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110.
- Quigg, M. Circadian rhythms: Interactions with seizures and epilepsy. Epilepsy Res. 2000, 42, 43–55.
- Pavlova, M.K.; Shea, S.A.; Scheer, F.A.; Bromfield, E.B. Is there a circadian variation of epileptiform abnormalities in idiopathic generalized epilepsy? Epilepsy Behav. 2009, 16, 461–467.
- Hofstra, W.A. The circadian rhythm and its interaction with human epilepsy: A review of literature. Sleep Med. Rev. 2009, 13, 413–420.
- Pavlova, M.K.; Shea, S.A.; Bromfield, E.B. Day/night patterns of focal seizures. Epilepsy Behav. 2004, 5, 44–49.
- Pung, T.; Schmitz, B. Circadian rhythm and personality profile in juvenile myoclonic epilepsy. Epilepsia 2006, 47, 111–114.
- Durazzo, T.; Spencer, S.; Duckrow, R.; Novotny, E.; Spencer, D.; Zaveri, H. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 2008, 70, 1265–1271.
- Lemmer, B.; Labrecque, G. Chronopharmacology and chronotherapeutics: Definitions and concepts. Chronobiol. Int. 1987, 4, 319–329.
- Manganaro, S.; Loddenkemper, T.; Rotenberg, A. The Need for Antiepileptic Drug Chronotherapy to Treat Selected Childhood Epilepsy Syndromes and Avert the Harmful Consequences of Drug Resistance. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573516685883.
- Loddenkemper, T.; Vendrame, M.; Zarowski, M.; Gregas, M.; Alexopoulos, A.V.; Wyllie, E.; Kothare, S.V. Circadian patterns of pediatric seizures. Neurology 2011, 76, 145–153.
- Guilhoto, L.M.; Loddenkemper, T.; Vendrame, M.; Bergin, A.; Bourgeois, B.F.; Kothare, S.V. Higher evening antiepileptic drug dose for nocturnal and early-morning seizures. Epilepsy Behav. 2011, 20, 334–337.
- Autret, A.; Lucas, B.; Laffont, F.; Bertrand, P.; Degiovanni, E.; De Toffol, B. Two distinct classifications of adult epilepsies: By time of seizures and by sensitivity of the interictal paroxysmal activities to sleep and waking. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 211–218.
- Karoly, P.; Goldenholz, D.; Freestone, D.; Moss, R.; Grayden, D.; Theodore, W.; Cook, M. Circadian and circaseptan rhythms in human epilepsy: A retrospective cohort study. Lancet Neurol. 2018, 17, 977–985.
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; Van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685.
- Mirzoev, A.; Bercovici, E.; Stewart, L.S.; Cortez, M.A.; Snead, O.C., 3rd; Desrocher, M. Circadian profiles of focal epileptic seizures: A need for reappraisal. Seizure 2012, 21, 412–416.
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521.
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530.
- Labate, A.; Ambrosio, R.; Gambardella, A.; Sturniolo, M.; Pucci, F.; Quattrone, A. Usefulness of a morning routine EEG recording in patients with juvenile myoclonic epilepsy. Epilepsy Res. 2007, 77, 17–21.
- Hofstra, W.A.; Spetgens, W.P.; Leijten, F.S.; Van Rijen, P.C.; Gosselaar, P.; Van Der Palen, J.; De Weerd, A.W. Diurnal rhythms in seizures detected by intracranial electrocorticographic monitoring: An observational study. Epilepsy Behav. 2009, 14, 617–621.
- Hofstra, W.A.; Grootemarsink, B.E.; Dieker, R.; Van Der Palen, J.; De Weerd, A.W. Temporal distribution of clinical seizures over the 24-h day: A retrospective observational study in a tertiary epilepsy clinic. Epilepsia 2009, 50, 2019–2026.
- Karafin, M.; St Louis, E.K.; Zimmerman, M.B.; Sparks, J.D.; Granner, M.A. Bimodal ultradian seizure periodicity in human mesial temporal lobe epilepsy. Seizure 2010, 19, 347–351.
- Spencer, D.C.; Sun, F.T.; Brown, S.N.; Jobst, B.C.; Fountain, N.B.; Wong, V.S.; Mirro, E.A.; Quigg, M. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia 2016, 57, 1495–1502.
- Daley, J.T.; DeWolfe, J.L. Sleep, Circadian Rhythms, and Epilepsy. Curr. Treat. Options Neurol. 2018, 20, 47.
- Leguia, M.G.; Andrzejak, R.G.; Rummel, C.; Fan, J.M.; Mirro, E.A.; Tcheng, T.K.; Rao, V.R.; Baud, M.O. Seizure Cycles in Focal Epilepsy. JAMA Neurol. 2021, 78, 454–463.
- Baud, M.O.; Kleen, J.K.; Mirro, E.A.; Andrechak, J.C.; King-Stephens, D.; Chang, E.F.; Rao, V.R. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun. 2018, 9, 88.
- Gregg, N.M.; Nasseri, M.; Kremen, V.; Patterson, E.E.; Sturges, B.K.; Denison, T.J.; Brinkmann, B.H.; Worrell, G.A. Circadian and multiday seizure periodicities, and seizure clusters in canine epilepsy. Brain Commun. 2020, 2, fcaa008.
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447.
- Wang, H. Perfect timing: A Nobel Prize in Physiology or Medicine for circadian clocks. Sci. Bull. 2018, 63, 398–401.
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41.
- Lee, Y.; Field, J.M.; Sehgal, A. Circadian Rhythms, Disease and Chronotherapy. J. Biol. Rhythm. 2021, 36, 503–531.
- Buijs, F.N.; León-Mercado, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181.
- Shin-ichi, T.I.; Kawamura, H. Characteristics of a circadian pacemaker in the suprachiasmatic nucleus. J. Comp. Physiol. 1982, 146, 153–160.
- Schwartz, W.J.; Reppert, S.M.; Eagan, S.M.; Moore-Ede, M.C. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983, 274, 184–187.
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9.
- Bilu, C.; Kronfeld-Schor, N. Effects of circadian phase and melatonin injection on anxiety-like behavior in nocturnal and diurnal rodents. Chronobiol. Int. 2013, 30, 828–836.
- Vivanco, P.; Ortiz, V.; Rol, M.A.; Madrid, J.A. Looking for the keys to diurnality downstream from the circadian clock: Role of melatonin in a dual-phasing rodent, Octodon degus. J. Pineal Res. 2007, 42, 280–290.
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99.
- Brown, S.A.; Azzi, A. Peripheral circadian oscillators in mammals. Handb. Exp. Pharmacol. 2013, 217, 45–66.
- Gerstner, J.R.; Smith, G.G.; Lenz, O.; Perron, I.J.; Buono, R.J.; Ferraro, T.N. BMAL1 controls the diurnal rhythm and set point for electrical seizure threshold in mice. Front. Syst. Neurosci. 2014, 8, 121.
- Li, P.; Fu, X.; Smith, N.A.; Ziobro, J.; Curiel, J.; Tenga, M.J.; Martin, B.; Freedman, S.; Cea-Del Rio, C.A.; Oboti, L.; et al. Loss of CLOCK Results in Dysfunction of Brain Circuits Underlying Focal Epilepsy. Neuron 2017, 96, 387–401.
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569.
- Sato, T.K.; Yamada, R.G.; Ukai, H.; Baggs, J.E.; Miraglia, L.J.; Kobayashi, T.J.; Welsh, D.K.; Kay, S.A.; Ueda, H.R.; Hogenesch, J.B. Feedback repression is required for mammalian circadian clock function. Nat. Genet. 2006, 38, 312–319.
- Gachon, F.; Fonjallaz, P.; Damiola, F.; Gos, P.; Kodama, T.; Zakany, J.; Duboule, D.; Petit, B.; Tafti, M.; Schibler, U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004, 18, 1397–1412.
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192.
- Ukai-Tadenuma, M.; Yamada, R.G.; Xu, H.; Ripperger, J.A.; Liu, A.C.; Ueda, H.R. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell 2011, 144, 268–281.
- Minami, Y.; Ode, K.L.; Ueda, H.R. Mammalian circadian clock: The roles of transcriptional repression and delay. Handb. Exp. Pharmacol. 2013, 217, 359–377.
- Lipton, J.O.; Yuan, E.D.; Boyle, L.M.; Ebrahimi-Fakhari, D.; Kwiatkowski, E.; Nathan, A.; Guttler, T.; Davis, F.; Asara, J.M.; Sahin, M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 2015, 161, 1138–1151.
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; Hogenesch, J.B.; Cao, R. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369.
- Liu, D.; Stowie, A.; De Zavalia, N.; Leise, T.; Pathak, S.S.; Drewes, L.R.; Davidson, A.J.; Amir, S.; Sonenberg, N.; Cao, R. mTOR signaling in VIP neurons regulates circadian clock synchrony and olfaction. Proc. Natl. Acad. Sci. USA 2018, 115, E3296–E3304.
- Khan, S.; Nobili, L.; Khatami, R.; Loddenkemper, T.; Cajochen, C.; Dijk, D.J.; Eriksson, S.H. Circadian rhythm and epilepsy. Lancet Neurol. 2018, 17, 1098–1108.
- Sahar, S.; Zocchi, L.; Kinoshita, C.; Borrelli, E.; Sassone-Corsi, P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS ONE 2010, 5, e8561.
- Sharma, S.K.; Dakshinamurti, K. Seizure activity in pyridoxine-deficient adult rats. Epilepsia 1992, 33, 235–247.
- Wu, H.; Liu, Y.; Liu, L.; Meng, Q.; Du, C.; Li, K.; Dong, S.; Zhang, Y.; Li, H.; Zhang, H. Decreased expression of the clock gene Bmal1 is involved in the pathogenesis of temporal lobe epilepsy. Mol Brain 2021, 14, 113.
- Zhang, T.; Yu, F.; Xu, H.; Chen, M.; Chen, X.; Guo, L.; Zhou, C.; Xu, Y.; Wang, F.; Yu, J.; et al. Dysregulation of REV-ERBα impairs GABAergic function and promotes epileptic seizures in preclinical models. Nat. Commun. 2021, 12, 1216.
- Ng, M.; Pavlova, M. Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res. Treat. 2013, 2013, 932790.
- Frauscher, B.; Von Ellenrieder, N.; Dubeau, F.; Gotman, J. EEG desynchronization during phasic REM sleep suppresses interictal epileptic activity in humans. Epilepsia 2016, 57, 879–888.
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263.
- Bazhenov, M.; Timofeev, I.; Steriade, M.; Sejnowski, T. Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J. Neurophysiol. 2000, 84, 1076–1087.
- Minecan, D.; Natarajan, A.; Marzec, M.; Malow, B. Relationship of epileptic seizures to sleep stage and sleep depth. Sleep 2002, 25, 899–904.
- Jin, B.; Hu, W.; Ye, L.; Krishnan, B.; Aung, T.; Jones, S.E.; Najm, I.M.; Alexopoulos, A.V.; Zhang, K.; Zhu, J.; et al. Small Lesion Size Is Associated with Sleep-Related Epilepsy in Focal Cortical Dysplasia Type II. Front. Neurol. 2018, 9, 106.
- Da Silva Lourenço, C.; Tjepkema-Cloostermans, M.C.; Van Putten, M. Machine learning for detection of interictal epileptiform discharges. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2021, 132, 1433–1443.
- De Berardis, D.; Orsolini, L.; Serroni, N.; Girinelli, G.; Iasevoli, F.; Tomasetti, C.; Mazza, M.; Valchera, A.; Fornaro, M.; Perna, G. The role of melatonin in mood disorders. ChronoPhysiology Ther. 2015, 5, 65.
- Schapel, G.J.; Beran, R.G.; Kennaway, D.L.; McLoughney, J.; Matthews, C.D. Melatonin response in active epilepsy. Epilepsia 1995, 36, 75–78.
- Bazil, C.W.; Short, D.; Crispin, D.; Zheng, W. Patients with intractable epilepsy have low melatonin, which increases following seizures. Neurology 2000, 55, 1746–1748.
- Molina-Carballo, A.; Acuna-Castroviejo, D.; Rodriguez-Cabezas, T.; Munoz-Hoyos, A. Effects of febrile and epileptic convulsions on daily variations in plasma melatonin concentration in children. J. Pineal Res. 1994, 16, 1–9.
- Kothare, S.V.; Kaleyias, J. Sleep and epilepsy in children and adolescents. Sleep Med. 2010, 11, 674–685.
- Dell, K.L.; Payne, D.E.; Kremen, V.; Maturana, M.I.; Gerla, V.; Nejedly, P.; Worrell, G.A.; Lenka, L.; Mivalt, F.; Boston, R.C.; et al. Seizure likelihood varies with day-to-day variations in sleep duration in patients with refractory focal epilepsy: A longitudinal electroencephalography investigation. EClinicalMedicine 2021, 37, 100934.
- Staniszewska, A.; Maka, A.; Religioni, U.; Olejniczak, D. Sleep disturbances among patients with epilepsy. Neuropsychiatr. Dis. Treat. 2017, 13, 1797–1803.
- Leschziner, G. Seizures and Sleep: Not such strange bedfellows. Adv. Clin. Neurosci. Rehabil. 2022, 21, 19–21.
- Bazil, C.W.; Castro, L.H.; Walczak, T.S. Reduction of rapid eye movement sleep by diurnal and nocturnal seizures in temporal lobe epilepsy. Arch. Neurol. 2000, 57, 363–368.
- Gelinas, J.N.; Khodagholy, D.; Thesen, T.; Devinsky, O.; Buzsáki, G. Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat. Med. 2016, 22, 641–648.
- Mekky, J.F.; Elbhrawy, S.M.; Boraey, M.F.; Omar, H.M. Sleep architecture in patients with Juvenile Myoclonic Epilepsy. Sleep Med. 2017, 38, 116–121.
- Kalsbeek, A.; Van Der Spek, R.; Lei, J.; Endert, E.; Buijs, R.M.; Fliers, E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol. Cell. Endocrinol. 2012, 349, 20–29.
- Androulakis, I.P. Circadian rhythms and the HPA axis: A systems view. WIREs Mech. Dis. 2021, 13, e1518.
- Sen, A.; Sellix, M.T. The Circadian Timing System and Environmental Circadian Disruption: From Follicles to Fertility. Endocrinology 2016, 157, 3366–3373.
- Shao, S.; Zhao, H.; Lu, Z.; Lei, X.; Zhang, Y. Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology. Endocrinology 2021, 162, bqab117.
- Fawley, J.A.; Pouliot, W.A.; Dudek, F.E. Epilepsy and reproductive disorders: The role of the gonadotropin-releasing hormone network. Epilepsy Behav. 2006, 8, 477–482.
- Peng, B.W.; Li, X.J.; Wu, W.X.; Zeng, Y.R.; Liao, Y.T.; Hou, C.; Liang, H.C.; Zhang, W.; Wang, X.Y.; Chen, W.X. The Possible Role of Hypothalamus-Pituitary-Adrenal Dysfunction in Epileptic Spasms. Seizure 2020, 81, 145–150.
- Basu, T.; Maguire, J.; Salpekar, J.A. Hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Neurosci. Lett. 2021, 746, 135618.
- Musiek, E.S.; Fitzgerald, G.A. Molecular clocks in pharmacology. Handb. Exp. Pharmacol. 2013, 217, 243–260.
- Lu, D.; Wang, Z.; Wu, B. Pharmacokinetics-based Chronotherapy. Curr. Drug. Metab. 2022, 23, 2–7.
- Ruben, M.D.; Smith, D.F.; FitzGerald, G.A.; Hogenesch, J.B. Dosing time matters. Science 2019, 365, 547–549.
- Nicolas, J.M.; Bouzom, F.; Hugues, C.; Ungell, A.L. Oral drug absorption in pediatrics: The intestinal wall, its developmental changes and current tools for predictions. Biopharm. Drug Dispos. 2017, 38, 209–230.
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150.
- Henriksson, E.; Huber, A.L.; Soto, E.K.; Kriebs, A.; Vaughan, M.E.; Duglan, D.; Chan, A.B.; Papp, S.J.; Nguyen, M.; Afetian, M.E.; et al. The Liver Circadian Clock Modulates Biochemical and Physiological Responses to Metformin. J. Biol. Rhythm. 2017, 32, 345–358.
- Fauteck, J.; Schmidt, H.; Lerchl, A.; Kurlemann, G.; Wittkowski, W. Melatonin in epilepsy: First results of replacement therapy and first clinical results. Biol. Signals Recept. 1999, 8, 105–110.
