Primary malignant brain tumors are the most common solid neoplasm in childhood. Despite recent advances, many children affected by aggressive or metastatic brain tumors still present poor prognosis, therefore the development of more effective therapies is urgent. Cancer stem cells (CSCs) have been discovered and isolated in both pediatric and adult patients with brain tumors (e.g., medulloblastoma, gliomas and ependymoma). CSCs are a small clonal population of cancer cells responsible for brain tumor initiation, maintenance and progression, displaying resistance to conventional anticancer therapies. CSCs are characterized by a specific repertoire of surface markers and intracellular specific pathways. These unique features of CSCs biology offer the opportunity to build therapeutic approaches to specifically target these cells in the complex tumor bulk.
- nanoparticles
- stem cells
- cancer
1. Nanoparticles and Target Therapy
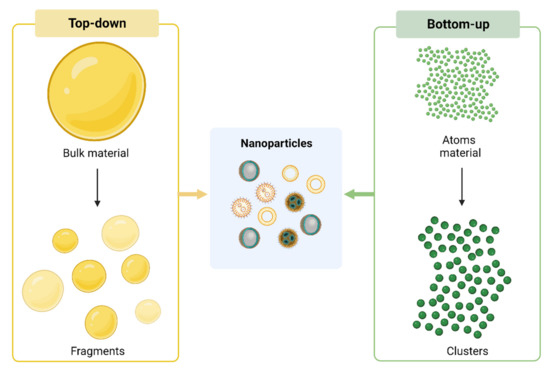
1.1. Synthesis of Nanoparticles
1.2. Mechanisms of Action
1.3. Passive Targeting
1.4. Active Targeting
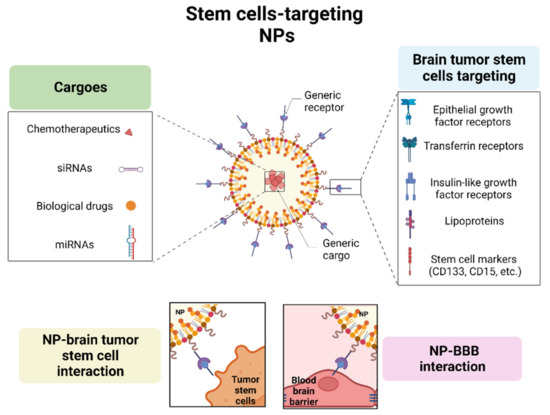
1.4.1. Epidermal Growth Factor Receptor
| Targeting Receptors | Type of NPs | Application | Target Cells | References |
|---|---|---|---|---|
| EGFR | NPs functionalized with Ang2 and EP-1 | Drug delivery | Endothelial cells of BBB (Ang2) and tumor cells (EP-1) | [12] |
| EGF-modified Au NP–Pc 4 | Delivery of photosensitizer silicon phthalocyanine | Tumor cells | [18] | |
| NPs conjugated to an EGFR antibody (Panitumumab/Vectibix) |
Drug delivery | Tumor cells | [1] | |
| Magnetic NPs conjugated to an EGFR deletion mutant (EGFRvIII) antibody | Magnetic resonance imaging | Tumor cells | [4] | |
| TfR | Tf-conjugated nanoparticles | Drug delivery | Tumor cells | [19][20][21][22][23][24] |
| Tf-conjugated nanoparticles | Drug delivery | Glioma stem cells and non-stem cells | [17] | |
| Tf-conjugated nanoparticles | Drug delivery | Glioma stem cells and non-stem cells | [19][20][21][22][23][24] | |
| IGFR | NPs functionalized with anti-insulin receptor antibody 83-14 | Drug delivery | BBB | [25] |
| NPs functionalized with anti-insulin receptor monoclonal antibody (29B4) | Drug delivery | BBB | [26] | |
| Lipoproteins | Gold-liposome nanoparticles conjugated with ApoE and RVG | RNAi delivery | Tumor cells (ApoE and RVG) and brain endothelium (RVG) | [27] |
| Nano-LDL particles | Drug delivery |
Tumor cells | [27] | |
| NPs conjugated to Angiopep-2 | Drug delivery |
BBB and tumor cells | [24] | |
| High-density lipoprotein nanoparticles | Intrinsic activity |
MB cells and stem cells | [27] |
1.4.2. Transferrin Receptor
1.4.3. Insulin Receptor
1.4.4. Lipoprotein
2. Immunotherapy and Nanoparticles in Pediatric Brain Tumors
3. Advanced Pre-Clinical Models to Study Nanoparticles in Pediatric Brain Tumors
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871.
- Shafey, A.M.E. Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339.
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576.
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392.
- Benjamin, L.E.; Golijanin, D.; Itin, A.; Pode, D.; Keshet, E. Selective Ablation of Immature Blood Vessels in Established Human Tumors Follows Vascular Endothelial Growth Factor Withdrawal. J. Clin. Investig. 1999, 103, 159–165.
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771.
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784.
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano Objects 2019, 19, 100370.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112.
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193.
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20.
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Enhanced Blood-Brain-Barrier Penetrability and Tumor-Targeting Efficiency by Peptide-Functionalized Poly(Amidoamine) Dendrimer for the Therapy of Gliomas. Nanotheranostics 2019, 3, 311–330.
- Cheng, Y.; Meyers, J.D.; Agnes, R.S.; Doane, T.L.; Kenney, M.E.; Broome, A.-M.; Burda, C.; Basilion, J.P. Addressing Brain Tumors with Targeted Gold Nanoparticles: A New Gold Standard for Hydrophobic Drug Delivery? Small Weinh. Bergstr. Ger. 2011, 7, 2301–2306.
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in Human Nanotechnology Doxorubicin Delivery System to Target Epidermal Growth Factor Receptors in Recurrent Glioblastoma. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2015, 22, 1889–1894.
- Schmitt, R.R.; Mahajan, S.D.; Pliss, A.; Prasad, P.N. Small Molecule Based EGFR Targeting of Biodegradable Nanoparticles Containing Temozolomide and Cy5 Dye for Greatly Enhanced Image-Guided Glioblastoma Therapy. Nanomed. Nanotechnol. Biol. Med. 2022, 41, 102513.
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328.
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII Antibody-Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Res. 2010, 70, 6303–6312.
- Ertas, Y.N.; Abedi Dorcheh, K.; Akbari, A.; Jabbari, E. Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances. Nanomaterials 2021, 11, 1755.
- Liu, G.; Mao, J.; Jiang, Z.; Sun, T.; Hu, Y.; Jiang, Z.; Zhang, C.; Dong, J.; Huang, Q.; Lan, Q. Transferrin-Modified Doxorubicin-Loaded Biodegradable Nanoparticles Exhibit Enhanced Efficacy in Treating Brain Glioma-Bearing Rats. Cancer Biother. Radiopharm. 2013, 28, 691–696.
- Ren, W.; Chang, J.; Yan, C.; Qian, X.; Long, L.; He, B.; Yuan, X.; Kang, C.; Betbeder, D.; Sheng, J.; et al. Development of Transferrin Functionalized Poly(Ethylene Glycol)/Poly(Lactic Acid) Amphiphilic Block Copolymeric Micelles as a Potential Delivery System Targeting Brain Glioma. J. Mater. Sci. Mater. Med. 2010, 21, 2673–2681.
- Xiao, W.; Wang, Y.; Zhang, H.; Liu, Y.; Xie, R.; He, X.; Zhou, Y.; Liang, L.; Gao, H. The Protein Corona Hampers the Transcytosis of Transferrin-Modified Nanoparticles through Blood-Brain Barrier and Attenuates Their Targeting Ability to Brain Tumor. Biomaterials 2021, 274, 120888.
- Sheykhzadeh, S.; Luo, M.; Peng, B.; White, J.; Abdalla, Y.; Tang, T.; Mäkilä, E.; Voelcker, N.H.; Tong, W.Y. Transferrin-Targeted Porous Silicon Nanoparticles Reduce Glioblastoma Cell Migration across Tight Extracellular Space. Sci. Rep. 2020, 10, 2320.
- Luo, M.; Lewik, G.; Ratcliffe, J.C.; Choi, C.H.J.; Mäkilä, E.; Tong, W.Y.; Voelcker, N.H. Systematic Evaluation of Transferrin-Modified Porous Silicon Nanoparticles for Targeted Delivery of Doxorubicin to Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 33637–33649.
- Liu, D.-Z.; Cheng, Y.; Cai, R.-Q.; Wang Bd, W.-W.; Cui, H.; Liu, M.; Zhang, B.; Mei, Q.-B.; Zhou, S.-Y. The Enhancement of SiPLK1 Penetration across BBB and Its Anti Glioblastoma Activity in Vivo by Magnet and Transferrin Co-Modified Nanoparticle. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 991–1003.
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283.
- Sharma, G.; Lakkadwala, S.; Modgil, A.; Singh, J. The Role of Cell-Penetrating Peptide and Transferrin on Enhanced Delivery of Drug to Brain. Int. J. Mol. Sci. 2016, 17, 806.
- Grafals-Ruiz, N.; Rios-Vicil, C.I.; Lozada-Delgado, E.L.; Quiñones-Díaz, B.I.; Noriega-Rivera, R.A.; Martínez-Zayas, G.; Santana-Rivera, Y.; Santiago-Sánchez, G.S.; Valiyeva, F.; Vivas-Mejía, P.E. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. Int. J. Nanomed. 2020, 15, 2809–2828.
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res. 2018, 8, 916–931.
- Rosager, A.M.; Sørensen, M.D.; Dahlrot, R.H.; Hansen, S.; Schonberg, D.L.; Rich, J.N.; Lathia, J.D.; Kristensen, B.W. Transferrin Receptor-1 and Ferritin Heavy and Light Chains in Astrocytic Brain Tumors: Expression and Prognostic Value. PLoS ONE 2017, 12, e0182954.
- Pardridge, W.M.; Chou, T. Mathematical Models of Blood-Brain Barrier Transport of Monoclonal Antibodies Targeting the Transferrin Receptor and the Insulin Receptor. Pharmaceuticals 2021, 14, 535.
- Sun, T.; Wu, H.; Li, Y.; Huang, Y.; Yao, L.; Chen, X.; Han, X.; Zhou, Y.; Du, Z. Targeting Transferrin Receptor Delivery of Temozolomide for a Potential Glioma Stem Cell-Mediated Therapy. Oncotarget 2017, 8, 74451–74465.
- Kim, S.-S.; Rait, A.; Rubab, F.; Rao, A.K.; Kiritsy, M.C.; Pirollo, K.F.; Wang, S.; Weiner, L.M.; Chang, E.H. The Clinical Potential of Targeted Nanomedicine: Delivering to Cancer Stem-like Cells. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 278–291.
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of Nanoparticles through the Blood-Brain Barrier for Imaging and Therapeutic Applications. Nanoscale 2014, 6, 2146–2152.
- Wu, D.; Yang, J.; Pardridge, W.M. Drug Targeting of a Peptide Radiopharmaceutical through the Primate Blood-Brain Barrier in Vivo with a Monoclonal Antibody to the Human Insulin Receptor. J. Clin. Investig. 1997, 100, 1804–1812.
- Dieu, L.-H.; Wu, D.; Palivan, C.G.; Balasubramanian, V.; Huwyler, J. Polymersomes Conjugated to 83-14 Monoclonal Antibodies: In Vitro Targeting of Brain Capillary Endothelial Cells. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2014, 88, 316–324.
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the Insulin Receptor: Nanoparticles for Drug Delivery across the Blood-Brain Barrier (BBB). J. Drug Target. 2011, 19, 125–132.
- Pawar, S.; Koneru, T.; McCord, E.; Tatiparti, K.; Sau, S.; Iyer, A.K. LDL Receptors and Their Role in Targeted Therapy for Glioma: A Review. Drug Discov. Today 2021, 26, 1212–1225.
- Hayavi, S.; Halbert, G.W. Synthetic Low-Density Lipoprotein, a Novel Biomimetic Lipid Supplement for Serum-Free Tissue Culture. Biotechnol. Prog. 2005, 21, 1262–1268.
- Nikanjam, M.; Blakely, E.A.; Bjornstad, K.A.; Shu, X.; Budinger, T.F.; Forte, T.M. Synthetic Nano-Low Density Lipoprotein as Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. Int. J. Pharm. 2007, 328, 86–94.
- Nikanjam, M.; Gibbs, A.R.; Hunt, C.A.; Budinger, T.F.; Forte, T.M. Synthetic Nano-LDL with Paclitaxel Oleate as a Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. J. Control. Release Off. J. Control. Release Soc. 2007, 124, 163–171.
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of Multifunctional Peptide Collaborated and Docetaxel Loaded Lipid Nanoparticles for Antiglioma Therapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2018, 132, 168–179.
- Bell, J.B.; Rink, J.S.; Eckerdt, F.; Clymer, J.; Goldman, S.; Thaxton, C.S.; Platanias, L.C. HDL Nanoparticles Targeting Sonic Hedgehog Subtype Medulloblastoma. Sci. Rep. 2018, 8, 1211.
- Poot, E.; Maguregui, A.; Brunton, V.G.; Sieger, D.; Hulme, A.N. Targeting Glioblastoma through Nano- and Micro-Particle-Mediated Immune Modulation. Bioorg. Med. Chem. 2022, 72, 116913.
- Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A.; Quintarelli, C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics 2022, 12, 173.
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O.; et al. Blood-Brain Barrier Permeable Nano Immunoconjugates Induce Local Immune Responses for Glioma Therapy. Nat. Commun. 2019, 10, 3850.
- Meng, L.; Wang, C.; Lu, Y.; Sheng, G.; Yang, L.; Wu, Z.; Xu, H.; Han, C.; Lu, Y.; Han, F. Targeted Regulation of Blood-Brain Barrier for Enhanced Therapeutic Efficiency of Hypoxia-Modifier Nanoparticles and Immune Checkpoint Blockade Antibodies for Glioblastoma. ACS Appl. Mater. Interfaces 2021, 13, 11657–11671.
- Voth, B.L.; Pelargos, P.E.; Barnette, N.E.; Bhatt, N.S.; Chen, C.H.J.; Lagman, C.; Chung, L.K.; Nguyen, T.; Sheppard, J.P.; Romiyo, P.; et al. Intratumor Injection of CCL21-Coupled Vault Nanoparticles Is Associated with Reduction in Tumor Volume in an in Vivo Model of Glioma. J. Neurooncol. 2020, 147, 599–605.
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic Programming of Macrophages to Perform Anti-Tumor Functions Using Targeted MRNA Nanocarriers. Nat. Commun. 2019, 10, 3974.
- Azambuja, J.H.; Schuh, R.S.; Michels, L.R.; Gelsleichter, N.E.; Beckenkamp, L.R.; Iser, I.C.; Lenz, G.S.; de Oliveira, F.H.; Venturin, G.; Greggio, S.; et al. Nasal Administration of Cationic Nanoemulsions as CD73-SiRNA Delivery System for Glioblastoma Treatment: A New Therapeutical Approach. Mol. Neurobiol. 2020, 57, 635–649.
- Kadiyala, P.; Gregory, J.V.; Lowenstein, P.R.; Lahann, J.; Castro, M.G. Targeting Gliomas with STAT3-Silencing Nanoparticles. Mol. Cell. Oncol. 2021, 8, 1870647.
- Lenzen, A.; Cole, L.; Lauing, K.L.; Zhai, L.; Ladomersky, E.; Lulla, R.R.; Hashizume, R.; Stegh, A.; Wainwright, D.A. Immu-24. Immunotherapeutic Nanotechnology Targeting Ido1 for Pediatric Diffuse Intrinsic Pontine Glioma. Neuro Oncol. 2018, 20, i103.
- Sayour, E.; Mendez-Gomez, H.; Grippin, A.; De Leon, G.; Stover, B.; Flores, C.; Pham, C.; Mitchell, D. Mbrs-02. Personalized Immunotherapy with Translatable Rna Nanoparticles Targeting Medulloblastoma. Neuro Oncol. 2018, 20, i128–i129.
- Mendez-Gomez, H.; McGuiness, J.; Grippin, A.; Weidert, F.; Carrera-Justiz, S.; Mitchell, D.; Sayour, E. Immu-13. Customizable Multi-Lamellar Rna-Nanoparticles for Pediatric Glioma. Neuro Oncol. 2021, 23, i29–i30.
- Perini, G.; Giulimondi, F.; Palmieri, V.; Augello, A.; Digiacomo, L.; Quagliarini, E.; Pozzi, D.; Papi, M.; Caracciolo, G. Inhibiting the Growth of 3D Brain Cancer Models with Bio-Coronated Liposomal Temozolomide. Pharmaceutics 2021, 13, 378.
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A Predictive Microfluidic Model of Human Glioblastoma to Assess Trafficking of Blood-Brain Barrier-Penetrant Nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119.
- Kim, J.S.; Shin, D.H.; Kim, J.-S. Dual-Targeting Immunoliposomes Using Angiopep-2 and CD133 Antibody for Glioblastoma Stem Cells. J. Control. Release Off. J. Control. Release Soc. 2018, 269, 245–257.
