Prostate cancer (PC) is a common malignancy and is one of the leading causes of cancer-related death in men worldwide. Osteosarcoma (OS) is the most common bone cancer, representing 20–40% of all bone malignancy cases. Cancer metastasis is a process by which malignant tumor cells detach from the primary tumor site via a cascade of processes and migrate to secondary sites through the blood circulation or lymphatic system to colonize and form secondary tumors. PC has a specific affinity to the bone based on the “seed and soil” theory; once PC reach the bone, it becomes incurable. Several studies have identified long noncoding RNAs (lncRNAs) as potential targets for cancer therapy or as diagnostic and prognostic biomarkers. The dysregulation of various lncRNAs has been found in various cancer types, including PC, OS, and metastasis.
- prostate cancer
- osteosarcoma
- metastasis
- lncRNAs
1. Introduction
2. Oncogenic lncRNAs in PC and OS Malignancy
2.1. Differentiation Antagonizing Non-Protein Coding RNA
3.1. Differentiation Antagonizing Non-Protein Coding RNA
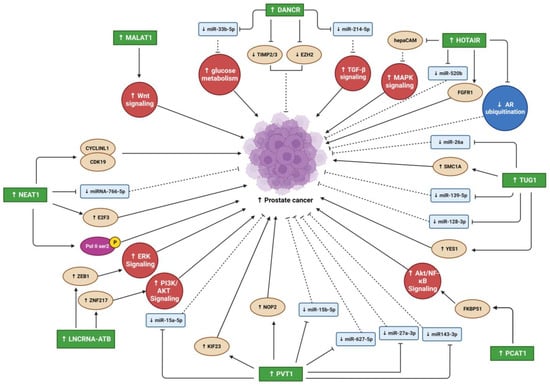
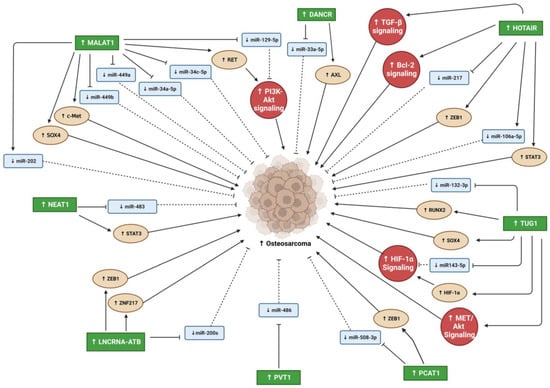
Differentiation antagonizing non-protein coding RNA (DANCR) is an oncogenic lncRNA with a crucial role in the progression of many cancer types [27][76]. Jia et al. reported that DANCR is highly upregulated in PC, with PC invasion and migration promoted by DANCR in vitro [28][35]. Moreover, metastasis was enhanced by DANCR overexpression in a xenograft prostate tumor mouse model [28][35]. Therefore, the DANCR lncRNA might be a target for PC metastasis prevention [28][35]. DANCR overexpression led to miRNA 33b-5p (miR-33b-5p) downregulation via sponging, promoting glycolysis in a Taxol-resistant cell line (PC3-TXR) [29][36]. DANCR knockdown in PC cell lines also slowed and reduced cell growth and migration [29][36]. Low DANCR expression in PC cells increased their sensitivity to Taxol (a chemotherapy drug) [29][36]. Similarly, Deng et al. found higher DANCR expression in two PC cell lines (DU145 and PC-3M) and higher DANCR levels in the serum of PC patients compared to controls [30][37]. Moreover, DANCR overexpression activated TGF-β, increasing PC proliferation and migration and decreasing apoptosis via its sponging of miR-214-5p [30][37]. The DANCR/miR-214-5p/TGF-β regulatory axis’s effects on PC progression may be a new therapeutic target for PC [30][37]. DANCR overexpression promoted OS cell line proliferation, migration, and invasion and promoted tumor growth and metastasis in vivo [31][38]. Through miR-33a-5p sponging, DANCR enhanced AXL receptor tyrosine kinase (AXL) expression, increasing the activity of downstream targets of the AXL/protein kinase B (AKT) axis [31][38]. Therefore, abnormal AXL activity regulates tumor cell self-regeneration and contributes to poor OS patient prognoses [31][38]. Notably, the AXL signaling pathway appears important in bone tumor pathogenesis due to its regulation of bone cancer cell colony formation and EMT [31][38].2.2. Metastasis Associated Lung Adenocarcinoma Transcript 1
3.2. Metastasis Associated Lung Adenocarcinoma Transcript 1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) overexpression has been found in various cancer cell lines and tumors [32][77]. MALAT1 is a significant osteogenic differentiation promoter for bone cells [33][78]. Because MALAT1 has been found in plasma and tissue samples of patients with PC, it has been suggested as an early PC biomarker [32][34][77,79]. However, because MALAT1 is expressed in diverse cell types, it is a less specific diagnostic biomarker for distinguishing between tumor origins [32][77]. However, to improve diagnostic sensitivity and accuracy, MALAT1 might be used in clinical diagnosis as a complementary biomarker for hematic cancer detection [32][34][77,79]. MALAT1 upregulation has been reported in human PC tissues and cell lines (22RV1 and LNCaP-AI) [35][40]. MALAT1 suppression reduced cancer cell growth, invasion, migration, and colony formation and elevated cell cycle arrest and apoptosis [35][40].2.3. Nuclear Enriched Abundant Transcript 1
3.3. Nuclear Enriched Abundant Transcript 1
Nuclear enriched abundant transcript 1 (NEAT1) expression is upregulated in various human malignancies, including PC [36][80]. NEAT1 increased cancer cell proliferation and invasion in vitro and in vivo, and its expression levels were highly associated with metastasis [22]. Wen et al. reported that high N6-methyladenosine (m6A) levels in NEAT1 enhanced cyclin L1 (CCNL1) and cyclin-dependent kinase 19 (CDK19) binding, increasing DNA polymerase II (Pol II) Ser2 phosphorylation and promoting PC-associated-bone metastasis, and correlated with poor PC prognosis [37][45]. Furthermore, this novel oncogenic regulatory network comprising CCNL1, CDK19, and NEAT1 could be a therapeutic target for bone metastasis [37][45]. NEAT1 is the most upregulated lncRNA in PC and is associated with PC progression [36][80]. Its overexpression in PC causes resistance to androgen deprivation therapy, suggesting that androgen receptor antagonists combined with NEAT1 targeting treatment may have synergistic antitumor effects on PC [36][80]. The regulatory network comprising NEAT1, miR-766-5p, and E2F transcription factor 3 (E2F3) enhances PC progression [38][46]. E2F3 is a downstream target of miR-766-5p, whose expression is activated by NEAT1 sponging of miR-766-5p [38][46]. NEAT1 suppression inhibited PC cell proliferation, invasion, and migration and stimulated apoptosis and cell cycle arrest [38][46].2.4. HOX Transcript Antisense RNA
3.4. HOX Transcript Antisense RNA
HOX transcript antisense RNA (HOTAIR) is an oncogenic lncRNA believed to be a significant diagnostic and prognostic biomarker for several tumors [39][81]. It was one of the first lncRNAs associated with tumorigenesis and metastasis [24]. How HOTAIR induces PC invasion and metastasis remains unclear. However, a recent study identified the hepatocellular adhesion molecule (hepaCAM) gene as a novel HOTAIR target crucial for promoting PC invasiveness, which inhibits mitogen-activated protein kinase (MAPK) signaling [40][49]. HOTAIR led to abnormal MAPK pathway activation by recruiting polycomb repressive complex 2 (PRC2) to the hepaCAM promoter, reducing its expression [40][49]. HOTAIR was overexpressed in four human OS cell lines (U2OS, MG63, Saos-2, and SW1353) compared to a human osteoblast cell line hFOB [41][52]. In addition, the tumor suppressor miR-217 was sponged by HOTAIR, increasing zinc finger E-box binding homeobox 1 (ZEB1) expression [41][52]. HOTAIR silencing in two OS cell lines (MG-63 and SW1353) suppressed their proliferation, migration, and invasion [41][52]. These findings suggest that the HOTAIR/miR-217/ZEB1 regulatory axis plays an important role in OS progression [41][52]. Zheng et al. showed that HOTAIR upregulation in the MG-63 cell line increased proliferation and decreased apoptosis [42][53]. Moreover, HOTAIR knockdown upregulated tumor necrosis factor-alpha (TNF-α) and tumor protein p53 (TP53) but downregulated B-cell lymphoma 2 (BCL2) and TGF-β [42][53]. Moreover, HOTAIR upregulation enhanced the resistance of multidrug chemotherapy cisplatin (DDP) in DDP-resistant OS tissues and cell lines (Saos2/DDP, MG-63/DDP, and U2OS/DDP) [43][54]. HOTAIR sponged miR-106a-5p, upregulating STAT3, increasing cell proliferation and invasion, and decreasing apoptosis [43][54].2.5. Taurine Upregulated Gene 1
3.5. Taurine Upregulated Gene 1
Taurine-upregulated gene 1 (TUG1) is an oncogenic lncRNA overexpressed in several cancer types [44][82]. TUG1 was significantly overexpressed and miR-26a was significantly under expressed in patient PC tissues compared to normal prostate tissues [45][56]. Moreover, TUG1 overexpression promoted cell proliferation, invasion due to EMT regulation, and metastasis in vitro [45][56]. TUG1 knockdown increased apoptosis and reduced proliferation in two PC cell lines (DU145 and PC3) [45][56]. The study suggested that TUG1 overexpression reduced miR-26a levels through sponging to promote PC progression and metastasis. Therefore, TUG1 may be a potential target for PC treatment [45][56]. TUG1 was significantly overexpressed in OS patient tissues and cell lines [46][47][48][49][59,60,61,62]. Additionally, OS patients had higher plasma TUG1 levels than healthy controls, showing poor prognoses and low survival [46][59]. Furthermore, TUG1 overexpression upregulated RUNX family transcription factor 2 (RUNX2) in two OS cell lines (MG-63 and U2OS), significantly enhancing their proliferation, migration, and invasion [46][59]. OS development of cisplatin (DDP)-resistance is a serious problem limiting the effectiveness of chemotherapy treatment [47][60]. TUG1 overexpression was higher in two DDP-resistant OS cell lines (Saos2/DDP and MG-63/DDP) than in their parent cell lines (MG-63 and Saos2) [47][60]. Moreover, TUG1 knockdown in vitro reduced OS cell DDP resistance through increased apoptosis and inhibited their MET/AKT signaling pathway [47][60].2.6. PC Associated Transcript 1
3.6. PC Associated Transcript 1
PC-associated transcript 1 (PCAT1) is a lncRNA with an oncogenic effect on PC and several other human solid and hematological cancers [50][83]. Shang et al. found higher PCAT1 overexpression in CRPC patient tissues than in androgen-dependent PC (ADPC) patient tissues [51][63]. Its overexpression correlated with increased cancer progression and reduced survival rate in CRPC patients [51][63]. An important regulatory network comprising FK506-binding protein 51 (FKBP51), which acts as a scaffolding protein, regulates PH domain leucine-rich repeat protein phosphatase (PHLPP) and inhibitory kappa B kinase α (IKKα) functions [51][63]. Increased PCAT1 bound directly to FKBP51 disrupted the PHLPP/FKBP51/IKKα network [51][63]. PCAT1 was abnormally overexpressed in OS patient tissues and cell lines [52][53][64,65]. Moreover, tissues from patients with metastatic OS had significantly higher PCAT1 expression than tissues from patients with non-metastatic OS [52][64]. PCAT1 upregulation was associated with poor prognosis, tumor metastasis, and advanced clinical stage in OS patients [52][64].2.7. LncRNA-Activated by TGF-β
3.7. LncRNA-Activated by TGF-β
LncRNA-activated by TGF-β (lncRNA-ATB) is an oncogenic lncRNA involved in metastasis cascade upregulation and EMT stimulation in several cancer types [54][84]. Xu et al. reported lncRNA-ATB overexpression in PC tissues and cell lines (PC3 and DU145) [55][66]. Furthermore, PC cell line proliferation was reduced by lncRNA-ATB knockdown, inhibiting cell cycle regulatory proteins (cyclins E1 (CCNE1) and D1 (CCND1)) expression [55][66]. LncRNA-ATB overexpression promoted EMT by reducing cell adhesion and polarization protein expression (CDH1 and tight junction protein 1 (TJP1/ZO-1)) in epithelial cells [55][66].2.8. Plasmacytoma Variant Translocation 1
3.8. Plasmacytoma Variant Translocation 1
Plasmacytoma variant translocation 1 (PVT1) is an oncogenic lncRNA overexpressed in many solid tumors and some hematological cancers [56][57][85,86]. Wu et al. found PVT1 abnormally overexpressed in PC patient tissues and cell lines (22RV1 and DU145), increasing PC progression by PVT1 sponging miR-15a-5p and inducing the expression of its downstream target kinesin family member 23 (KIF23) [58][68]. PVT1 knockdown in both cell lines downregulated KIF23 via miR-15a-5p, decreasing cell proliferation, invasion, and migration and increasing apoptosis. PVT1 knockdown also suppressed tumor progression in vivo [58][68]. Liu et al. analyzed PVT1 next-generation RNA sequencing (RNA-Seq) data and clinical information for 498 PC patients from the TCGA-PRAD database, finding that those with higher PVT1 expression had poor prognoses and higher mortality than those with lower PVT1 expression [59][69]. PVT1 expression was higher in PC metastatic tissues than in primary PC tissues. PVT1 was also upregulated in four PC cell lines (VCaP, PC3, DU145, and 22RV1) [60][70]. PVT1 knockdown inhibited DU145 cell migration and invasion, reduced tumor progression and metastasis, and increased survival time in vivo [60][70]. PVT1 overexpression promoted PC progression and metastasis by sponging several miRNAs (miR-15b-5p, miR-27a-3p, miR-143-3p, and miR-627-5p) [60][70]. PVT1 was overexpressed in OS patient tissues and cell lines [61][62][71,72]. Yan et al. found PVT1 highly upregulated in OS patients with metastasis compared to patients without metastasis [61][71]. PVT1 overexpression promoted migration, invasion, and metastasis in two OS cell lines (HOS and 143B). In addition, miR-486 was downregulated in OS patient tissues due to sponging by PVT1 [61][71]. Patients with higher PVT1 expression were recently reported to have worse prognoses [62][72]. PVT1 knockdown suppressed proliferation, invasion, and migration in OS MG-63 and SW1353 cells [62][72].34. Conclusions
In the past and even now, cancer and metastases remain life threating situations. Despite all research that reveled many factors and pathways that are involved in development, prognosis, and metastases, the regulatory mechanism that control these pathways still remain unknown. Recent studies focused on lncRNA as regulatory modulators of many biological processes contrary to the prior belief that lncRNAs are transcriptional noise. They have vital roles in many cellular processes and the pathogeneses of many diseases, including cancer (Figure 12 and Figure 23). While numerous studies have reported the oncogenic effects of lncRNAs in different cancer types (e.g., PC, OS, and other solid tumor metastasis), the mechanisms underlying their involvement in cancer progression and metastasis are highly complex and remain incompletely understood. Many studies have suggested that some oncogenic lncRNAs may be therapeutic targets and biomarkers for cancer diagnosis and prognosis. However, further studies on lncRNA/miRNA/mRNA regulatory network mechanisms and their downstream targets are essential for understanding their involvement in oncogenesis. In addition, more studies are needed to understand the contributions of oncogenic lncRNAs in the interaction between PC and bone microenvironment that will enhance our understanding of PC-associated bone metastasis, which may lead to the design of novel therapeutic strategies to treat this devastating disease.