Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aura Rusu and Version 2 by Dean Liu.
Antibacterial fluoroquinolones (FQs) are frequently used in treating infections. However, the value of FQs is debatable due to their association with severe adverse effects (AEs). The Food and Drug Administration (FDA) issued safety warnings concerning their side-effects in 2008, followed by the European Medicine Agency (EMA) and regulatory authorities from other countries.
- fluoroquinolones
- antibacterial quinolones
- adverse effects
1. The Main FQs with Clinical Importance
The classification into generations based on the spectrum of activity and therapeutic indications is the most used. New compounds are acquired from one generation to another with a broader spectrum of activity (Table 1) and improved pharmacokinetic properties [1]. Among the representatives of the fourth generation are topical FQs (ophthalmic and otic), such as besifloxacin [2] and finafloxacin [3].
New FQs (including a nonfluorinated QN) were approved in India, Japan, South Korea, and Taiwan [4]. These representatives will be addressed in a subsequent section [3][5][6][7].
Table 1. QNs and FQs approved by the FDA and EMA from the perspective of the antibacterial spectrum and the main indications (EMA—European Medicine Agency, FDA—USA Food and Drug Administration, FQs—fluoroquinolones, QNs—antibacterial quinolones).
| QNs/FQs | 1st Generation | 2nd Generation | 3rd Generation | 4th Generation |
|---|---|---|---|---|
| Nalidixic Acid | Ciprofloxacin, Nadifloxacin 1, Norfloxacin, Ofloxacin, Pefloxacin | Gatifloxacin 2, Levofloxacin |
Besifloxacin 2, Delafloxacin, Finafloxacin 3, Moxifloxacin | |
| Antibacterial spectrum | Enterobacteria. No activity against Gram-positive bacteria. |
Enterobacteriaceae; some atypical pathogens; Pseudomonas aeruginosa (only Ciprofloxacin); some Gram-positive bacteria (including Streptococcus pneumoniae), moderate activity against Staphylococcus aureus (Ciprofloxacin, Norfloxacin, Ofloxacin, Pefloxacin) Staphylococcus aureus ((MRSA) and coagulase-negative staphylococci), aerobic Gram-negative and anaerobic pathogens (Nadifloxacin 1) |
Broad-spectrum, including Staphylococcus aureus, Streptococcus species, and Gram-negative pathogens (Gatifloxacin 2) Enterobacteriaceae; Atypical pathogens; Streptococcus pneumoniae, penicillin-resistant (Levofloxacin) |
Streptococcus pneumoniae, Staphylococcus epidermidis, Staphylococcus aureus, Hemophilus influenzae, Moraxella catarrhalis, Corynebacterium spp. (Besifloxacin 2) Broad-spectrum (including methicillin-resistant Staphylococcus aureus) (Delafloxacin) Broad-spectrum activity (Finafloxacin 3) Enterobacteriaceae; atypical pathogens; Pseudomonas aeruginosa; Streptococci; Staphylococcus aureus methicillin-sensitive; anaerobic pathogens (Moxifloxacin) |
| Indications | Uncomplicated urinary tract infections (UTI) | Uncomplicated and complicated UTI, pyelonephritis, sexually transmitted diseases, prostatitis, respiratory tract infections, skin, soft tissues, bones, and joint infections (Ciprofloxacin, Norfloxacin, Ofloxacin, Pefloxacin) Acne vulgaris and other skin infections (Nadifloxacin 1). |
Bacterial conjunctivitis due to susceptible pathogens (Gatifloxacin 2) Acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial) (Levofloxacin) |
Bacterial conjunctivitis (Besifloxacin 2) Bacterial skin and skin structure infections (Delafloxacin) Acute otitis externa (Finafloxacin 3) Sexually transmitted diseases, prostatitis, skin and tissue infections, acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), intra-abdominal infections, and gynecological infections (Moxifloxacin) |
| References | [8][9] | [10][11] | [12][13][14] | [12][15][16] |
1 Topical (skin), 2 Topical (ophthalmic), 3 Topical (otic) administration.
2. Essential Chemical Characteristics
Structural characterization of FQs (older and newer representatives) was recently described in two other papers by our group of authors [1][4]. Essential structural elements of FQs will be briefly highlighted below. FQs are based on quinoline nucleus (ciprofloxacin, norfloxacin, pefloxacin, moxifloxacin, delafloxacin. etc.). Still, some compounds are 1,8-naphthyridine derivatives (e.g., nalidixic acid and zabofloxacin) or tricyclic compounds that include a quinoline nucleus (ofloxacin/levofloxacin, nadifloxacin) (Figure 12) [4][17][18][19].
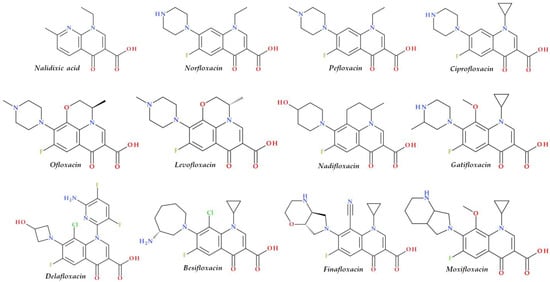
Figure 12. Chemical structures of FQs used in therapy and approved by the EMA and FDA (EMA—European Medicine Agency, FDA—Food and Drug Administration, FQs—fluoroquinolones).
The N1 position is involved in pharmacokinetic properties and overall potency. Substitution with a cyclopropyl moiety increased the activity against Gram-negative bacteria (e.g., ciprofloxacin). Other substituents were less beneficial or associated with severe AEs (e.g., 2,4-difluorophenyl in temafloxacin) [19][20][21][22]. A 6-amino-3,5-difluoropyridinyl moiety enlarges the delafloxacin’s molecular surface and is responsible for the activity against Gram-positive pathogens [23][24].
The C2 position is optimal without substitution, and a larger substituent may hinder the C3 and C4 positions [20]. A carboxyl group in the C3 position and an oxo(keto) group in the C4 position are essential for interacting with the DNA bases and the enzyme DNA gyrase [19][20][25][26]. Small radicals substituted at the C5 position (e.g., methyl or amino) may increase activity against Gram-positive bacteria, but currently, FQs used in therapy have no substituents in this position [19][20]. The number of halogen substituents on the basic nucleus varies. Many compounds contain a single fluorine atom in the C6 position. The substitution with a fluorine atom increased the potency of the FQs [19][20]. Besifloxacin contains two different halogens in the structure (fluorine in the C6 position and chlorine in the C8 position) [27]. Other representatives have three fluorine atoms (e.g., lascufloxacin) or three fluorine and one chlorine atom (e.g., delafloxacin). Nemonoxacin is a non-fluorinated QN, approved in Taiwan in 2014 [28]. Halogen substitutions lead to increased permeability, decreased solubility, and increased lipophilicity of the compounds [29][30].
The C7 position controls the pharmacokinetics and antibacterial activity of FQs. A five- or six-membered nitrogen heterocycle is optimal, such as piperazine (second-generation FQs), pyrrolo-piperidine (e.g., moxifloxacin), hexahydro-1H-azepine (e.g., besifloxacin), 3-hydroxyazetidine (e.g., delafloxacin), and pyrrolo-oxazine (e.g., finafloxacin). The substitution with a piperazine nucleus increased activity against Gram-negative bacteria (especially for the second-generation FQs). Other heterocycles increased activity against Gram-positive bacteria [4][20][21]. In general, the C8 position controls pharmacokinetic properties and activity against anaerobic bacteria. A beneficial C8 substituent is the methoxy group found in moxifloxacin and gatifloxacin and the recent representatives, lascufloxacin and nemonoxacin (Figure 23) [19].
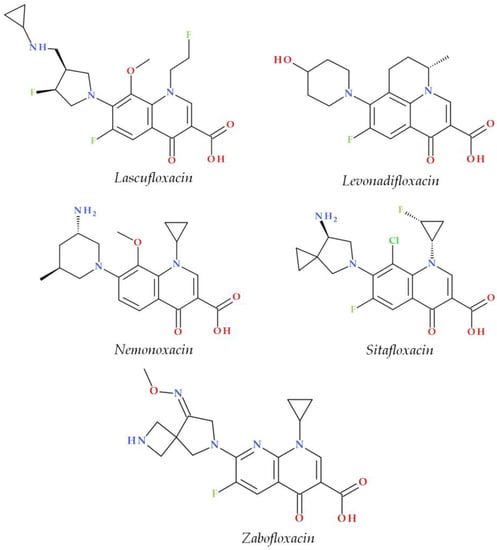
Figure 23.
Chemical structures of the recently approved systemic antibacterial (fluoro)quinolones in the countries where they were produced.
Chlorine substitution at the C8 position increased the antimicrobial potency of besifloxacin by acting on the two target enzymes, DNA gyrase and topoisomerase IV [31]. The C8 cyano group in finafloxacin seems essential in activity against Gram-positive bacteria [3][15].
3. Mechanism of Action
Many papers concerning the FQs mechanism of action have already been published [18][25][32][33][34][35]. Antibacterial QNs act by inhibiting two enzymes involved in bacterial DNA replication, DNA gyrase and DNA topoisomerase IV. DNA gyrase introduces negative supercoils into DNA, an essential activity for the initiation of DNA replication. Topoisomerase IV removes the interlinking of daughter chromosomes (decatenation) to segregate chromosomes (and plasmids) into daughter cells at the end of a round of replication. The second function of topoisomerase IV (shared with the DNA gyrase) is to relax positive supercoils. In Gram-negative pathogens, the primary target is the DNA gyrase enzyme, while in Gram-positive pathogens, the primary target is the topoisomerase IV enzyme. Thus, the first FQs generations target only the DNA gyrase enzyme from Gram-negative bacteria [25][35][36][37]. Newer FQs representatives target both enzymes from Gram-negative and Gram-positive bacteria [33][38]. Due to FQ-enzyme-DNA complex formation, DNA replication is reversibly inhibited, and the DNA is cleaved in both strands [32][35]. Bacterial death occurs depending on the drug concentration [37]. At low concentrations, FQs block reversible DNA replication and transcription. Next, the inhibition of DNA and RNA synthesis occurs. Thus, the growth of bacteria will be inhibited, but only during FQs therapy [32]. A higher drug concentration (over the minimum inhibitory concentration (MIC)) leads to its binding to the topoisomerase-DNA cleavage complex. Bacterial death depends on the processing of the cleavage complex. The slow death of bacteria arises when the processing of the cleavage complex is missing, and DNA replication and transcription are blocked. Rapid death of bacteria occurs when the cleavage complex is processed, and the broken DNA repair no longer occurs. Due to chromosome fragmentation, the bacterial cell will quickly die [32][39]. These events can produce reactive oxygen species (ROS) and, consequently, more DNA breaks. The DNA damage induced by FQs can be restored with consequences concerning the survival of the bacterial cell [32].
Recent studies highlight the importance of ROS formation and FQs’ lethality [40][41]. It seems that ROS are the dominant factor in FQs’ lethality. ROS accumulation completes the primary DNA damage induced by FQs to kill bacterial cells [41][42]. Numerous studies regarding the increased oxidative stress state generated by the FQs treatment were discussed by Michalak et al. [43].
4. Safety Warnings concerning Emerging Serious AEs
Although the approved FQs are helpful in treating infections with sensitive germs, a significant disadvantage is the potential risk of associated severe AEs (involving muscles, tendons, or joints and the nervous system) [44][45]. The most reported severe AEs are tendon rupture (especially to the Achilles tendon), arthralgia, tendonitis, pain in extremities, gait disturbance, neuropathies associated with paresthesia, fatigue, memory impairment, depression, sleep disorders, impaired vision, hearing, taste and smell, phototoxicity, genotoxicity, QTc prolongation, hematological effect, hepatic eosinophilia effect, pulmonary interstitial eosinophilia, immunological side-effects, hypoglycemia, and CYP 450 inhibition [18][46]. Due to some severe AEs from those previously listed, many compounds have been withdrawn from therapy (Section 3.5). Thus, FQs are contraindicated in patients who have previously experienced side-effects during treatment with a (fluoro)quinolone antibiotic [46][47]. Since the risks exceed the benefits, the FDA and EMA have recently restricted the use of FQs in treating mild and uncomplicated infections, non-bacterial infections, preventing traveler’s diarrhea, and recurring lower UTI, unless other recommended antibacterial agents cannot be used [45][47][48][49]. Additionally, the FDA and EMA recommended that FQs should not be used as first-line therapies in treating acute sinusitis, bacterial infections among persons with chronic obstructive pulmonary disease (COPD), or UTIs, as the risks outweigh the benefits [46][49].
In 2016, the FDA mandated label revisions for all systemic FQs, along with a Black Box Warning about the possibility of irreversible AEs, including the muscles, joints, tendons, nerves, and central nervous system (CNS), that can occur together in the same patient (Table 2) [50][51]. Additionally, new warnings were made, and other parts of the FQs’ label were updated [50]. In 2018, the list of the approved FQs by the FDA comprised ciprofloxacin, delafloxacin, levofloxacin, gemifloxacin, moxifloxacin, and ofloxacin [52].
On 15 November 2018, the EMA completed an evaluation of significant, debilitating, and possibly irreversible AEs associated with QNs and FQs antibiotics administered orally, injectable, or inhaled, considering the experiences of patients, healthcare workers, and scientists. Following this evaluation, the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) has suggested that some medications, particularly those containing QNs, should be withdrawn from the market [1][2][6][7][9][11][12][13][14][16][21][22][23][24][26][29][30][31][35][37][38][39][40][41][42][43][44][45][46][47][49][50][51][52][53][54][55][56]. The QNs and FQs subject to these restrictions are: (a) cinoxacin, nalidixic acid, pipemidic acid (QNs), and (b) ciprofloxacin, flumequine, levofloxacin, lomefloxacin, moxifloxacin, norfloxacin, ofloxacin, pefloxacin, prulifloxacin, and rufloxacin (FQs) [53]. Instead, FQs should be used to treat illnesses when an antibiotic is required but other antibiotics are ineffective [56].
Table 2. FDA and EMA warnings concerning emerging severe AEs of QNs and FQs (AEs—adverse effects, EMA—European Medicine Agency, FDA—USA Food and Drug Administration, FQs—fluoroquinolones, QNs—antibacterial quinolones, Ref.—references).
| No. | Year | Regulatory Entity | Document | Title of Document | Targeted AEs | The Formulations/Administration Concerned | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2008 | FDA | FDA alert (8 July 2008) |
Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs Black Boxed Warning | Increased risk of tendinitis and tendon rupture | Formulations for systemic use (except ophthalmic or otic formulations) | [57][58][59] |
| 2 | 2011 | FDA | FDA alert (February 2011) |
Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs Black Boxed Warning? | Worsening symptoms of patients with myasthenia gravis | Formulations for systemic use | [48][60] |
| 3 | 2013 | FDA | FDA Drug Safety Communication (15 August 2013) |
FDA requires label changes to warn of the risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection | Side-effects of peripheral neuropathy | Formulations for systemic use except for ophthalmic or otic formulations | [61] |
| 4 | 2016 | FDA | FDA Drug Safety Communication (12 May 2016) |
FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side-effects that can occur together | Side-effects concerning tendons, muscles, joints, nerves, and CNS | Formulations for systemic use |
69] evaluated the risk of the common AEs associated with FQs. Additionally, this study compared the occurrence of AEs related to FQs and the occurrence of the AEs associated with other antimicrobial agents. Prescribing FQs led to a significantly higher occurrence of gastrointestinal and CNS side-effects compared to other antimicrobial agents (e.g., macrolides and cefuroxime axetil). However, FQs were associated with fewer gastrointestinal and CNS side-effects versus fosfomycin and the combination of trimethoprim-sulfamethoxazole. In addition, FQs were not related to skin-related AEs [69][70][71].
Regulations from other countries (exclusive of the USA and EU) concerning FQ-associated AEs are discussed below.
Canada. There are five FQs (oral and injectable) approved in Canada by different companies: ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin, and ofloxacin. In 2016, Health Canada changed the labels of oral FQs due to reported cases of patients developing retinal detachment, which has become a significant concern regarding FQs. Health Canada authority emphasizes the necessity of seeing a medical professional if the patients report eye problems during or following the FQs’ administration [72]. In addition, at the beginning of 2017, other safety labeling updates informed Canadians and medical professionals about the possibility of persistent or severe AEs, such as tendinopathy, peripheral neuropathy, and CNS disorders [73].
United Kingdom. Ciprofloxacin, moxifloxacin, levofloxacin, and ofloxacin are the FQs approved for therapy in the United Kingdom. In 2019, the Medicines and Healthcare products Regulatory Agency (MHRA) advised that FQs should not be prescribed to treat non-severe or non-bacterial diseases unless other antibiotics are ineffective. Additionally, following a review of the above-listed severe AEs of these drugs, all FQs were limited, with added health labeling concerns [74]. In 2020, a new safety warning for patients at risk for heart valve regurgitation was announced, stating that FQs should be used only following a rigorous benefit–risk evaluation of other alternative treatments [70].
Australia. Since 1976, Australia has expanded the regulatory standards for antimicrobial treatments among humans, and the use of QNs medications is known for its role as a backup antimicrobial agent. Every three years, a group of experts in infectious diseases evaluates the guidelines for antimicrobial administration in the population and hospitals. FQs are prescribed when necessary or as a better-suited therapy, such as in patients with severe CAP that have acute penicillin hypersensitivity, but in most situations, empirical regimens in national prescription guidelines advise the use of aminoglycosides, β-lactams, or macrolides [75]. There are three approved FQs in Australia: ciprofloxacin, norfloxacin, and moxifloxacin. In 2019, following the public announcements of the FDA and EMA regarding the severe AEs induced by FQs, the Therapeutic Goods Administration (TGA) began researching a rare but severe adverse event of aortic aneurysm associated with FQs [76]. During the TGA’s research, it was also decided to update the labeling of FQs to ensure that all products contain warnings concerning the potential AEs of dysglycemia and adverse mental reactions [77].
5. Withdrawal of Some FQs over Time
Although some new FQs representatives proved to have good antibacterial activity, a broader activity spectrum, or better pharmacokinetic properties compared to compounds of previous generations, they were associated with severe AEs that led to their withdrawal from the market. Approved FQs that were withdrawn after a few years of approval are alatrofloxacin/trovafloxacin, gatifloxacin, gemifloxacin, grepafloxacin, sparfloxacin, and temafloxacin (Table 3) [45][78].
Table 3. Examples of FQs withdrawn from therapy due to severe AEs (AEs—adverse effects, FQs—fluoroquinolones).
| No. | FQs (Generation) |
Manufacturer | Approval Year | Withdrawn Year |
Side-Effects | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fleroxacin (2nd) | Kyorin Pharmaceutical | 1981 | 1990 | CNS effects, phototoxicity | [44][79][80] | ||||||||
| 2 | Tosufloxacin (2nd) | Toyama Chemical | 1990 | 2006 | Thrombocytopenia, nephritis, toxic epidermal necrosis, eosinophilic pneumonitis | [44][81][82][83] | ||||||||
| 3 | Temafloxacin (2nd) | Abbott Laboratories | 1992 | 1992 | “Temafloxacin syndrome”: hemolytic-uremic syndrome | [8][22][44][84][85][86] | ||||||||
| 4 | Lomefloxacin (2nd) | Serle | 1992 | 1993 | CNS effects, phototoxicity | [87][88][89 | [62] | |||||||
| ] | 5 | 2016 | FDA | FDA Drug Safety Communication (26 July 2016) |
FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side-effects (safety labeling changes) | Side-effects involving nerves, the CNS, tendons, muscles, and joints | Formulations for systemic use | [48] | ||||||
| 5 | Sparfloxacin (3rd) | Mylan | 1996 | 2001 | QT prolongation, phototoxicity | [89][90][91][92][93][94] | 6 | 2018 | FDA | FDA (10 July 2018) |
FDA reinforces safety information about serious low blood sugar levels and mental health side-effects with fluoroquinolone antibiotics; requires label changes (warnings) | Serious risk of blood sugar drop and negative impact on mental health | ||
| 6 | Formulations for systemic use | Alatrofloxacin (3rd) | [ | Pfizer | 1997 | 2006 | Seizures, thrombocytopenia, hepatotoxicity |
[94][95][96]52] | ||||||
| 7 | 2018 | FDA | FDA Drug Safety Communication (20 December 2018) |
FDA warns about the increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients (safety announcement) | Higher risk of aortic dissections or ruptures of an aortic aneurysm | Formulations for systemic use | ||||||||
| 7 | Trovafloxacin (3rd) | [ | Pfizer | 63 | ] | |||||||||
| 1997 | 2000 | Hepatotoxicity | [ | 85 | ] | [94][97][98] | 8 | 2018 | EMA | EMA/668915/2018 (5 October 2018) |
Fluoroquinolone and quinolone antibiotics: PRAC recommends new restrictions on use following a review of disabling potentially long-lasting side-effects available online | Long-term adverse effects affecting tendons, bones, and the nervous system | Formulations for systemic and inhalation route | [53] |
| 8 | Grepafloxacin (3rd) | Glaxo | 1997 | 1999 | QT prolongation, fatal cardiotoxicity, gastrointestinal toxicity |
[85][94] | 9 | 2019 | EMA | EMA/175398/2019 (11 March 2019) |
Disabling and potentially permanent side-effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics | Side-effects involving the CNS, bones, muscles, joints, and tendons | Formulations for systemic and inhalation route | [46] |
| 10 | 2020 | EMA | EMA/Direct Healthcare Professional Communication (DHPC) (29 November 2020) |
DHPC: Systemic and inhaled FQs: risk of heart valve regurgitation/incompetence |
Risk of heart valve regurgitation/incompetence | Formulations for systemic and inhalation route | [64] |
As a result, the EMA’s PRAC and the FDA recommend restrictions on the prescribing of QNs and FQs due to potentially life-threatening side-effects, such as tendon rupture, musculoskeletal pain, and nerve damage (Table 2) [49]. Additionally, FQs should be contraindicated in patients who have already experienced substantial AEs from a (fluoro)quinolone regimen. FQs should be used with extreme caution in elderly patients, patients with renal illness, and those who have undergone an organ transplant, due to an increased risk of tendon rupture. Additionally, combining FQs and corticosteroids raises the risk of tendon rupture. Therefore, this combination should be avoided [46].
Etminam et al. [65] found that the FQs therapy could be associated with increased aortic and mitral regurgitation. In the same year, a cellular and molecular mechanism was documented concerning FQ-associated aortopathy [66]. Consequently, prescribing advice for specialists has been issued by the EMA and other countries (e.g., the United Kingdom). Systemic or inhaled FQs should be used only after following a rigorous benefit–risk evaluation of different treatments available in the case of individuals at risk for heart valve regurgitation [64][67]. The FDA has not issued any warnings or recommendations regarding increased aortic and mitral regurgitation associated with FQs.
However, in a recent study, Strange et al. [68] demonstrated that increased valvular regurgitation rates are not significantly associated with oral FQs. Therefore, more studies must confirm or deny the link between FQs and increased aortic and mitral regurgitation.
A comprehensive review and meta-analysis published by Tandan et al. [
Severe AEs such as hepatotoxicity, dysglycemia, Clostridium difficile infection, fatal arrhythmia due to QT prolongation, and severe hemolytic-uremic syndrome increased the risks of FQs administration and ultimately led to the withdrawal of some FQs on the market all over the world [49]. Only five FQs representatives are approved for systemic use in the USA market (ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, and delafloxacin) [45]. In 2017, the FQs were in the top 10 topics of the Division of Drug Information (DDI), which is closely connected with the FDA Center for Drug Evaluation and Research (CDER) [106]. In addition, a situation about systemic FQs associated with potential AEs events reported to the DDI in 2013–2017 highlights 2016 as the year with the most significant number of inquiries (703) [107].
References
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749.
- Singh, C.L.; Singh, A.; Kumar, S.; Majumdar, D.K. Besifloxacin the fourth generation fluoroquinolone: A review. J. Drug Deliv. Ther. 2014, 4, 39–44.
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506.
- Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289.
- Chang, L.-W.; Hsu, M.-C.; Zhang, Y.-Y. Nemonoxacin (Taigexyn®): A New Non-Fluorinated Quinolone; IntechOpen: London, UK, 2019; ISBN 978-1-78984-473-3.
- Tanaka, K.; Vu, H.; Hayashi, M. In Vitro Activities and Spectrum of Lascufloxacin (KRP-AM1977) against Anaerobes. J. Infect. Chemother. 2021, 27, 1265–1269.
- Bhatia, A.; Mastim, M.; Shah, M.; Gutte, R.; Joshi, P.; Kumbhar, D.; Periasamy, H.; Palwe, S.R.; Chavan, R.; Bhagwat, S.; et al. Efficacy and Safety of a Novel Broad-Spectrum Anti-MRSA Agent Levonadifloxacin Compared with Linezolid for Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Openlabel, Randomized Study. J. Assoc. Physicians India 2020, 68, 30–36.
- Ball, P. Quinolone Generations: Natural History or Natural Selection? J. Antimicrob. Chemother. 2000, 46, 17–24.
- Sweetman, S.C. (Ed.) Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2009; ISBN 978-0-85369-840-1.
- Beale, J.M., Jr.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Wolters Kluwer Health: Baltimore, MD, USA, 2010; ISBN 978-0-7817-7929-6.
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and Safety of Nadifloxacin for Bacterial Skin Infections: Results from Clinical and Post-Marketing Studies. Dermatol. Ther. 2014, 4, 233–248.
- Limberakis, C. Quinolone Antibiotics: Levofloxacin (Levaquin®), Moxifloxacin (Avelox®), Gemifloxacin (Factive®), and Garenoxacin (T-3811). In The Art of Drug Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–69. ISBN 978-0-470-13497-9.
- Cervantes, L.J.; Mah, F.S. Clinical Use of Gatifloxacin Ophthalmic Solution for Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2011, 5, 495–502.
- Drug Approval Package: Zymar (Gatifloxacin) NDA #021493. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021493_Zymar.cfm (accessed on 5 August 2022).
- McKeage, K. Finafloxacin: First Global Approval. Drugs 2015, 75, 687–693.
- Mah, F.S.; Sanfilippo, C.M. Besifloxacin: Efficacy and Safety in Treatment and Prevention of Ocular Bacterial Infections. Ophthalmol. Ther. 2016, 5, 1–20.
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 91, 1063–1065.
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739.
- Domagala, J.M.; Hagen, S.E. Structure-Activity Relationships of the Quinolone Antibacterials in the New Millennium: Some Things Change and Some Do Not. In Quinolone Antimicrobial Agents, 3rd ed.; American Society of Microbiology Press: Washington, DC, USA, 2003; pp. 3–18.
- Tillotson, G.S. Quinolones: Structure-Activity Relationships and Future Predictions. J. Med. Microbiol. 1996, 44, 320–324.
- Domagala, J.M. Structure-Activity and Structure-Side-Effect Relationships for the Quinolone Antibacterials. J. Antimicrob. Chemother. 1994, 33, 685–706.
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin Syndrome: Review of 95 Cases. Clin. Infect. Dis. 1994, 18, 946–950.
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.H.; Kufel, W.D. Clinical Review of Delafloxacin: A Novel Anionic Fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451.
- Bassetti, M.; Hooper, D.; Tillotson, G. Analysis of Pooled Phase 3 Safety Data for Delafloxacin in Acute Bacterial Skin and Skin Structure Infections. Clin. Infect. Dis. 2019, 68, S233–S240.
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320.
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones Structural and Medicinal Developments (2013–2018): Where Are We Now? Bioorganic Med. Chem. 2019, 27, 3005–3060.
- Totoli, E.G.; Nunes Salgado, H.R. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142.
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453.
- Lu, T.; Zhao, X.; Li, X.; Drlica-Wagner, A.; Wang, J.-Y.; Domagala, J.; Drlica, K. Enhancement of Fluoroquinolone Activity by C-8 Halogen and Methoxy Moieties: Action against a Gyrase Resistance Mutant of Mycobacterium Smegmatis and a Gyrase-Topoisomerase IV Double Mutant of Staphylococcus Aureus. Antimicrob. Agents Chemother. 2001, 45, 2703–2709.
- Thomas, G. Medicinal Chemistry: An Introduction, 2nd ed.; Wiley: Chicester, UK, 2008.
- Haas, W.; Sanfilippo, C.M.; Hesje, C.K.; Morris, T.W. Contribution of the R8 Substituent to the in Vitro Antibacterial Potency of Besifloxacin and Comparator Ophthalmic Fluoroquinolones. Clin. Ophthalmol. 2013, 7, 821–830.
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662.
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559.
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49, S73–S78.
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A.; Zhao, X. Quinolones: Action and Resistance Updated. Curr. Top Med. Chem. 2009, 9, 981–998.
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61.
- Cheng, G.; Hao, H.; Dai, M.; Liu, Z.; Yuan, Z. Antibacterial Action of Quinolones: From Target to Network. Eur. J. Med. Chem. 2013, 66, 555–562.
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445.
- Malik, M.; Zhao, X.; Drlica, K. Lethal Fragmentation of Bacterial Chromosomes Mediated by DNA Gyrase and Quinolones. Mol. Microbiol. 2006, 61, 810–825.
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071.
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive Oxygen Species Play a Dominant Role in All Pathways of Rapid Quinolone-Mediated Killing. J. Antimicrob. Chemother. 2020, 75, 576–585.
- Rodríguez-Rosado, A.I.; Valencia, E.Y.; Rodríguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Blázquez, J.; Rodríguez-Beltrán, J. Reactive Oxygen Species Are Major Contributors to SOS-Mediated Mutagenesis Induced by Fluoroquinolones. bioRxiv 2018, 428961.
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxidative Med. Cell. Longev. 2017, 2017, e8023935.
- Rubinstein, E. History of Quinolones and Their Side Effects. CHE 2001, 47, 3–8.
- Roberts, J.R. InFocus: Fluoroquinolone Side Effects Just Got Scarier. Emerg. Med. News 2018, 40, 26–27.
- Francisco, E.M. Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/news/disabling-potentially-permanent-side-effects-lead-suspension-restrictions-quinolone-fluoroquinolone (accessed on 12 August 2021).
- EMA. Quinolone- and Fluoroquinolone-Containing Medicinal Products. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 12 August 2021).
- Office of the Commissioner FDA Updates Warnings for Fluoroquinolone Antibiotics. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics (accessed on 12 August 2021).
- Gatti, M.; Bianchin, M.; Raschi, E.; De Ponti, F. Assessing the Association between Fluoroquinolones and Emerging Adverse Drug Reactions Raised by Regulatory Agencies: An Umbrella Review. Eur. J. Intern. Med. 2020, 75, 60–70.
- Center for Drug Evaluation and Research FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. FDA 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 16 September 2022).
- Aschenbrenner, D.S. The FDA Revises Boxed Warning For Fluoroquinolones-Again. Am. J. Nurs. 2016, 116, 22–23.
- Center for Drug Evaluation and Research. FDA Reinforces Safety Information about Serious Low Blood Sugar Levels and Mental Health Side Effects with Fluoroquinolone Antibiotics; Requires Label Changes. FDA 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-reinforces-safety-information-about-serious-low-blood-sugar-levels-and-mental-health-side (accessed on 20 September 2022).
- Francisco, E.M. Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends New Restrictions on Use Following Review of Disabling Potentially Long-Lasting Side Effects. Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review (accessed on 12 August 2021).
- Dassault Systèmes BIOVIA Draw for Academics. Available online: https://discover.3ds.com/biovia-draw-academic (accessed on 1 November 2022).
- Office of the Commissioner FDA. In Brief: FDA Warns That Fluoroquinolone Antibiotics Can Cause Aortic Aneurysm in Certain Patients. FDA 2019. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-fluoroquinolone-antibiotics-can-cause-aortic-aneurysm-certain-patients (accessed on 19 September 2022).
- EMA. Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 1–4 October 2018. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-1-4-october-2018 (accessed on 20 September 2022).
- Tanne, J.H. FDA Adds “Black Box” Warning Label to Fluoroquinolone Antibiotics. BMJ 2008, 337, 135.
- Waknine, Y. Fluoroquinolones Earn Black Box Warning for Tendon-Related Adverse Effects. Available online: https://www.medscape.com/viewarticle/577302 (accessed on 21 September 2022).
- Center for Drug Evaluation and Research Postmarket Drug Safety Information for Patients and Providers—Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs . Available online: http://wayback.archive-it.org/7993/20161022101528/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm (accessed on 21 September 2022).
- Jones, S.C.; Sorbello, A.; Boucher, R.M. Fluoroquinolone-Associated Myasthenia Gravis Exacerbation. Drug Saf. 2011, 34, 839–847.
- Center for Drug Evaluation and Research Drug Safety and Availability—FDA Drug Safety Communication: FDA Requires Label Changes to Warn of Risk for Possibly Permanent Nerve Damage from Antibacterial Fluoroquinolone Drugs Taken by Mouth or by Injection. Available online: http://wayback.archive-it.org/7993/20161022101530/http://www.fda.gov/Drugs/DrugSafety/ucm365050.htm (accessed on 21 September 2022).
- Center for Drug Evaluation and Research. FDA Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections; Warns about Disabling Side Effects That Can Occur Together. FDA 2016. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain (accessed on 21 September 2022).
- FDA. Drug Safety Communication FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics (accessed on 10 October 2022).
- EMA. Systemic and Inhaled Fluoroquinolones: Risk of Heart Valve Regurgitation/Incompetence. Available online: https://www.ema.europa.eu/en/medicines/dhpc/systemic-inhaled-fluoroquinolones-risk-heart-valve-regurgitationincompetence (accessed on 20 September 2022).
- Etminan, M.; Sodhi, M.; Ganjizadeh-Zavareh, S.; Carleton, B.; Kezouh, A.; Brophy, J.M. Oral Fluoroquinolones and Risk of Mitral and Aortic Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 1444–1450.
- Guzzardi, D.G.; Teng, G.; Kang, S.; Geeraert, P.J.; Pattar, S.S.; Svystonyuk, D.A.; Belke, D.D.; Fedak, P.W.M. Induction of Human Aortic Myofibroblast-Mediated Extracellular Matrix Dysregulation: A Potential Mechanism of Fluoroquinolone-Associated Aortopathy. J. Thorac. Cardiovasc. Surg. 2019, 157, 109–119.e2.
- Systemic and Inhaled Fluoroquinolones: Small Risk of Heart Valve Regurgitation; Consider Other Therapeutic Options First in Patients at Risk. Available online: https://www.gov.uk/drug-safety-update/systemic-and-inhaled-fluoroquinolones-small-risk-of-heart-valve-regurgitation-consider-other-therapeutic-options-first-in-patients-at-risk (accessed on 19 July 2022).
- Strange, J.E.; Holt, A.; Blanche, P.; Gislason, G.; Torp-Pedersen, C.; Christensen, D.M.; Hansen, M.L.; Lamberts, M.; Schou, M.; Olesen, J.B.; et al. Oral Fluoroquinolones and Risk of Aortic or Mitral Regurgitation: A Nationwide Nested Case-Control Study. Eur. Heart J. 2021, 42, 2899–2908.
- Tandan, M.; Cormican, M.; Vellinga, A. Adverse Events of Fluoroquinolones vs. Other Antimicrobials Prescribed in Primary Care: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 2018, 52, 529–540.
- Hook, E.W.; Golden, M.R.; Taylor, S.N.; Henry, E.; Tseng, C.; Workowski, K.A.; Swerdlow, J.; Nenninger, A.; Cammarata, S. Efficacy and Safety of Single-Dose Oral Delafloxacin Compared With Intramuscular Ceftriaxone for Uncomplicated Gonorrhea Treatment: An Open-Label, Noninferiority, Phase 3, Multicenter, Randomized Study. Sex Transm Dis 2019, 46, 279–286.
- Kurono, Y.; Kawauchi, H.; Hori, S.; Tateda, K.; Totsuka, K.; Asano, M.; Suzuki, K. Phase III Double-Blind Comparative Study of Lascufloxacin versus Levofloxacin in Patients with Sinusitis. Jpn. J. Chemother. 2020, 68, 68–80.
- Health Canada Summary Safety Review—Oral Fluoroquinolones—Assessing the Potential Risk of Retinal Detachment. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-oral-fluoroquinolones-assessing-potential-risk-retinal.html (accessed on 5 October 2022).
- Health Canada Summary Safety Review—Fluoroquinolones—Assessing the Potential Risk of Persistent and Disabling Side Effects. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-fluoroquinolones-assessing-potential-risk-persistent-disabling-effects.html (accessed on 5 October 2022).
- Medicines and Healthcare products Regulatory Agency Fluoroquinolone Antibiotics: New Restrictions and Precautions for Use Due to Very Rare Reports of Disabling and Potentially Long-Lasting or Irreversible Side Effects. Available online: https://www.gov.uk/drug-safety-update/fluoroquinolone-antibiotics-new-restrictions-and-precautions-for-use-due-to-very-rare-reports-of-disabling-and-potentially-long-lasting-or-irreversible-side-effects (accessed on 5 October 2022).
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460.
- Therapeutic Goods Administration (TGA) Fluoroquinolone Antibiotics and Risk of Aortic Aneurysm/Dissection. Available online: https://www.tga.gov.au/news/safety-updates/fluoroquinolone-antibiotics-and-risk-aortic-aneurysmdissection (accessed on 5 October 2022).
- Therapeutic Goods Administration (TGA) Update—Fluoroquinolone Antibiotics and Adverse Events. Available online: https://www.tga.gov.au/news/safety-updates/update-fluoroquinolone-antibiotics-and-adverse-events (accessed on 5 October 2022).
- Outterson, K.; Powers, J.H.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Kesselheim, A.S. Approval and Withdrawal of New Antibiotics and Other Antiinfectives in the U.S., 1980–2009. J. Law. Med. Ethics 2013, 41, 688–696.
- Bowie, W.R.; Willetts, V.; Jewesson, P.J. Adverse Reactions in a Dose-Ranging Study with a New Long-Acting Fluoroquinolone, Fleroxacin. Antimicrob. Agents Chemother. 1989, 33, 1778–1782.
- Geddes, A.M. Safety of Fleroxacin in Clinical Trials. Am. J. Med. 1993, 94, 201S–203S.
- Kimura, N.; Miyazaki, E.; Matsuno, O.; Abe, Y.; Tsuda, T. Drug-induced pneumonitis with eosinophilic infiltration due to tosufloxacin tosilate. J. Jpn. Respir. Soc. 1998, 36, 618–622.
- Choi, M.K.; Woo, H.Y.; Heo, J.; Cho, M.; Kim, G.H.; Song, G.A.; Kim, M.B. Toxic Epidermal Necrolysis Associated with Sorafenib and Tosufloxacin in a Patient with Hepatocellular Carcinoma. Ann. Dermatol. 2011, 23, S404–S407.
- Owens, R.C., Jr.; Ambrose, P.G. Antimicrobial Safety: Focus on Fluoroquinolones. Clin. Infect. Dis. 2005, 41, S144–S157.
- Aronson, J.K. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-444-53716-4.
- Mandell, L.A.; Ball, P.; Tillotson, G. Antimicrobial Safety and Tolerability: Differences and Dilemmas. Clin. Infect. Dis. 2001, 32, S72–S79.
- Finch, R.G. The Withdrawal of Temafloxacin. Drug Saf. 1993, 8, 9–11.
- Young, A.R.; Fakouhi, T.D.; Harrison, G.I.; Roniker, B.; Swabb, E.A.; Hawk, J.L.M. The UVR Wavelength Dependence for Lomefloxacin Photosensitization of Human Skin. J. Photochem. Photobiol. B Biol. 1996, 32, 165–170.
- Lowe, N.J.; Fakouhi, T.D.; Stern, R.S.; Bourget, T.; Roniker, B.; Swabb, E.A. Photoreactions with a Fluoroquinolone Antimicrobial: Evening versus Morning Dosing. Clin. Pharmacol. Ther. 1994, 56, 587–591.
- Petersen, U. Quinolone Antibiotics: The Development of Moxifloxacin. In Analogue-Based Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 315–370. ISBN 978-3-527-60800-3.
- Jaillon, P.; Morganroth, J.; Brumpt, I.; Talbot, G. Overview of Electrocardiographic and Cardiovascular Safety Data for Sparfloxacin. Sparfloxacin Safety Group. J. Antimicrob. Chemother. 1996, 37, 161–167.
- Rubinstein, E. Safety Profile of Sparfloxacin in the Treatment of Respiratory Tract Infections. J. Antimicrob. Chemother. 1996, 37, 145–160.
- Lipsky, B.A.; Dorr, M.B.; Magner, D.J.; Talbot, G.H. Safety Profile of Sparfloxacin, a New Fluoroquinolone Antibiotic. Clin. Ther. 1999, 21, 148–159.
- King, D.E.; Malone, R.; Lilley, S.H. New Classification and Update on the Quinolone Antibiotics. Am. Fam. Physician 2000, 61, 2741–2748.
- Qureshi, Z.P.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Stevenson, K.B.; Szeinbach, S.L. Market Withdrawal of New Molecular Entities Approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 772–777.
- Melvani, S.; Speed, B.R. Alatrofloxacin-Induced Seizures during Slow Intravenous Infusion. Ann. Pharmacother. 2000, 34, 1017–1019.
- Gales, B.J.; Sulak, L.B. Severe Thrombocytopenia Associated with Alatrofloxacin. Ann. Pharmacother. 2000, 34, 330–334.
- File, T.M., Jr.; Schlemmer, B.; Garau, J.; Cupo, M.; Young, C.; The 049 Clinical Study Group. Efficacy and Safety of Gemifloxacin in the Treatment of Community-Acquired Pneumonia: A Randomized, Double-Blind Comparison with Trovafloxacin. J. Antimicrob. Chemother. 2001, 48, 67–74.
- Pannu, H.K.; Gottlieb, L.; Fishman, E.K. Acute Liver Failure Due to Trovafloxacin: CT Findings. Emerg. Radiol. 2001, 8, 108–110.
- Stahlmann, R.; Schwabe, R. Safety Profile of Grepafloxacin Compared with Other Fluoroquinolones. J. Antimicrob. Chemother. 1997, 40, 83–92.
- Anderson, M.E.; Mazur, A.; Yang, T.; Roden, D.M. Potassium Current Antagonist Properties and Proarrhythmic Consequences of Quinolone Antibiotics. J. Pharmacol. Exp. Ther. 2001, 296, 806–810.
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Can. J. Infect. Dis. 1999, 10, 207–238.
- FDA. Determination That TEQUIN (Gatifloxacin) Was Withdrawn From Sale for Reasons of Safety or Effectiveness. Available online: https://www.federalregister.gov/documents/2008/09/09/E8-20938/determination-that-tequin-gatifloxacin-was-withdrawn-from-sale-for-reasons-of-safety-or (accessed on 14 September 2022).
- Mandell, L.; Tillotson, G. Safety of Fluoroquinolones: An Update. Can. J. Infect. Dis. 2002, 13, 54–61.
- EMA. Questions and Answers on the Withdrawal of the Marketing Authorisation Application for Factive Gemifloxacin. Available online: https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-withdrawal-marketing-authorisation-application-factive-gemifloxacin_en.pdf (accessed on 22 September 2022).
- EMA. Menarini International Operations Luxembourg Withdraws Its Marketing Authorisation Application for Factive (Gemifloxacin). Available online: https://www.ema.europa.eu/en/news/menarini-international-operations-luxembourg-withdraws-its-marketing-authorisation-application (accessed on 22 September 2022).
- Center for Drug Evaluation and Research. CDER Division of Drug Information. Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cder-division-drug-information (accessed on 22 September 2022).
- Molnar, D.M.; Kremzner, M.E. Fluoroquinolones: A Hot Topic for Pharmacists and the Food and Drug Administration’s Division of Drug Information. J. Am. Pharm. Assoc. 2019, 59, 13–16.
More
