Copper (Cu)-based composite coatings have been widely applied in all kinds of important industry fields due to their outstanding comprehensive properties. The preparation temperature of a composite coating is the key factor affecting the properties, so the cold spray (CS) technology is characterized by low-temperature solid-state deposition, which ensures its emergence as the most promising technology for preparing the Cu-based composite coatings. The CS process is achieved using high-pressure gas, during which the solid particles are regulated to impact the substrate at a supersonic speed and then deposited on the substrate surface to form a coating through severe plastic deformation. As a low-temperature solid-state deposition method, CS is characterized by a low deposition temperature, a low oxidation ratio of powder, a high deposition efficiency, little thermal influence on the substrate, etc., by which coatings with low porosity, high compactness, and controllable thickness can be prepared, establishing its indispensable position among coating preparation technologies.
1. Principle of Cold Spray Technology
In the late 1980s, Anatolii Papyrin, a scientist from the former Soviet Union, and his team conducted wind tunnel experiments and revealed a phenomenon that the effect of tracer particles on the target surface changes from erosion-targeted to deposition-targeted when the speed exceeds a certain critical value, contributing to the close binding between the tracer particles and the target. Based on this phenomenon, the concept of
cold spray (CS
) was put forward
[1][23]. The CS technology is highly valuable by virtue of its potential applications based on its performance in low-temperature solid-state deposition and its advantages in preparing coatings on metal surfaces, which has aroused worldwide interest, enabling its rapid development amo
[1]ng coating preparation technologies. Large quantities of studies have been conducted on the research and development of CS equipment, material system design, numerical simulation, deposition mechanism of coatings, and optimization of spray process parameters over the past two decades. These achievements have accelerated the experimental process of CS technology and made it a reliable surface-strengthening technology, substantially promoting the development and application of CS technology
[2][24].
CS is a novel technology for preparing a coating. During the CS, solid particles (particle size: 5–50 μm) undergo convergence and divergence through scaled de Laval nozzles under the action of a working gas (helium, nitrogen, air, gas mixtures, etc.) with a certain temperature and pressure to produce a supersonic biphasic flow (generally 300–1200 m/s), which collides with the substrate surface at high speed
[3][25]. When the impact velocity of the feedstock particles reaches or exceeds their critical deposition velocity (
vcrit), the solid particles impact the substrate to trigger “adiabatic shear instability (ASI)” at the interface to cause severe plastic flow deformation, thereby forming a coating on the substrate surface
[4][26].
As the CS technology has been continuously developed and gradually becomes mature, many new “CS + assistive technologies”, such as laser-assisted, shot-peening-assisted, magnetic-field-assisted, friction stir processing (FSP)-assisted, and pulse-assisted CS technologies, have been developed through the combination of the original single CS technology and other technologies
[5][27]. Different types of CS technologies can be adopted in light of the actual application requirements of different engineering projects, laying crucial foundations for the design, preparation, and application of high-performance Cu-based composite coatings.
2. Deposition Mechanism of Cold-Sprayed Coatings
The bonding mechanism of cold-sprayed coatings has always been a hot research topic. Up to now, no consensus has been reached on the deposition mechanism of coatings. Nowadays, the theory of ASI proposed by Assadi et al.
[6][28] is the most recognized coating deposition theory. The high-velocity impact of particles on the substrate causes severe plastic deformation at the interface of the impact, leading to the work hardening effect of the material. As the impact time is extremely short, most of the kinetic energy of the particles is converted into heat energy, and there is not enough time for energy transfer. Therefore, the heat energy causes a temperature rise, and results in the adiabatic softening of the material. If the impact velocity exceeds the critical velocity and the thermal softening effect overwhelms the work hardening effect, the plastic flow will appear on the metal surface, and then a metal jet will occur. At the same time, adiabatic shear instability (ASI) will occur on the particle surface. Grujici et al.
[7][29] conducted a more detailed analysis on the sensitivity of metallic materials to ASI during the CS based on the research of Assadi et al.
[6][28]. It is considered that the metal jet produced by ASI breaks and extrudes the oxide film on the contact interface between the particles and the corresponding substrate, which increases the contact area of the clean metal surface and facilitates the metallurgical bonding between the particles and the substrate. The fragmentation mechanism of oxide films proposed by Li et al.
[8][30] is also consistent with the viewpoints of Grujici
[7][29]. On the contrary, the latest research conducted by Hassani-Gangardj et al.
[9][31] revealed that ASI is not a prerequisite for particle bonding during the CS, and the adiabatic softening effect is not necessarily implicated in the generation of the metal jet. Hassani-Gangardj
[9][31] believed that, similar to explosive welding, a large pressure gradient at the particle interface induces the generation of the jet. These contradictions in science indicate that the deposition mechanism of cold-sprayed coatings has not yet been completely solved, which needs further research and exploration.
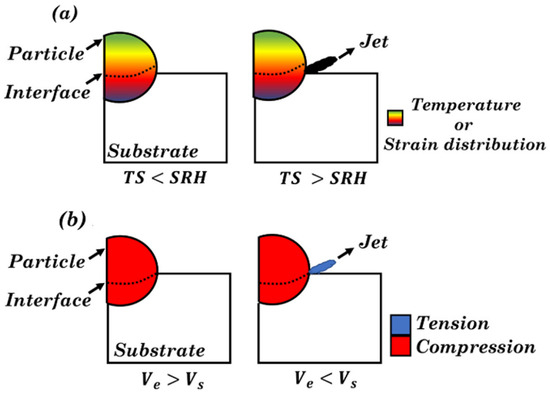
Fardan et al.
[10][32] intuitively elaborated the specific formation process of a metal jet in the cases of adiabatic shearing and jetting through model diagrams (
Figure 1). As shown in
Figure 1a, the impact of particles on the substrate leads to changes in the temperature and plastic strain, which induce a local temperature rise, causing plastic flow. This process explains the adiabatic shearing. When the rate of strain hardening is lower than that of thermal softening, a point of thermal instability known as ASI appears. At this point, it is found that jetting takes place at the periphery of the particles. However, Hassani-Gangardj
[9][31] argued that this bonding is attributed to hydrodynamic plasticity rather than ASI, and likewise, jetting is triggered by pressure release instead of ASI. At the time the particles impact the substrate, compressed pressure waves are generated and unevenly distributed in the particles and substrate (
Figure 1b). In the initial phase,
Ve is higher than
Vs, but at a threshold point, the hydrodynamic stress is higher than the flow strength of the material, and jetting occurs
[11][33]. According to other studies on hypervelocity impacts, such as shaped charge jetting, explosive welding, and droplet impact, jetting is induced by a pressure release, which is in accordance with the above-mentioned mechanism
[12][34].
Figure 1. Schematic representation of the jet formation
[10][32]. (
a) Adiabatic shear instability, first proposed by Assadi et al.
[6][28] and (
b) hydrodynamic plasticity proposed by Hassani et al.
[9][31]. TS = thermal softening, SRH = strain rate hardening,
Ve = edge velocity,
Vs = shock velocity.
On the basis of the ASI mechanism, researchers put forward four recognized bonding methods for coatings, namely, physical bonding, mechanical bonding, chemical bonding, and metallurgical bonding. Under the conditions of different materials and deposition processes, inter-particle bonding in coatings can be achieved by one or more of the above-mentioned methods. Hussain et al.
[13][35] believed that after the ASI of particles, the locally generated metal jet can crack and clear the oxide film on the substrate surface, exposing the clean particles and substrate surface. Under relatively high pressure, physical bonding can be formed between particles and the corresponding substrate or among particles under the action of electrostatic forces or Van der Waals forces. Moridi et al.
[14][36] explained the interface bonding mechanism in the particle deposition process using the mechanical bonding mechanism. They found that particles undergo the ASI and pressure results in the plastic deformation and metal jets of particles. After plastic deformation, the obtained flat particles are embedded with an uneven interface, which forms a mechanical interlocking structure, resulting in mechanical bonding. Xie et al.
[15][37] deposited a hard Ni coating on a soft aluminum (Al) substrate by the CS to clarify the bonding mechanism on the coating/substrate interface, and found the intermetallic compound Al
3Ni at the interface, demonstrating that the high temperature generated during the plastic deformation of particles will induce the chemical bonding between the coating and the corresponding substrate. Grujicic et al.
[16][38] held that the ASI is attributed to particle impacts, during which a large amount of heat energy will be generated. Local high temperatures can melt the substrate interface and strengthen the bonding at the coating/substrate interface, thus forming metallurgical bonding at the coating/substrate interface and inside the coating.
To sum up, physical bonding and mechanical bonding occur almost at the same time. Under the action of Van der Waals forces, mechanical bonding is manifested as a non-chemical reaction. Theoretically, hard particles are mechanically captured by soft substrate materials to form interlocks, thus triggering the mechanical bonding. Regarding the whole deposition process of coatings, interfacial bonding is dominated by mechanical bonding. It is generally considered that metallurgical bonding results from chemical bonding between dissimilar materials, which is always accomplished by atomic diffusion. Moreover, the strength and plasticity of metallurgical bonding are far superior to those of mechanical bonding. Nonetheless, the CS is characterized by low-temperature solid-state deposition, by which the coating deposition is realized by means of the plastic deformation of particles, therefore mechanical bonding is the main bonding form of cold-sprayed coatings.
3. Deposition Mechanism of Cold-Sprayed Cu-Based Composite Coatings
3.1. Deposition Mechanism of Cu-Based Metal Composite Coatings
The deposition mechanism of the interfacial bonding between metals and metal alloy coatings is attributed to the severe deformation process caused by the high-velocity impact of metal/metal alloy solid particles, which directly influences the interfacial bonding quality of the coatings and finally determines the bonding strength and mechanical properties of the coatings. The deposition mechanism of Cu-based metal composite coatings is identical to that of cold-sprayed metal and metal alloy coatings, but it is remarkably different when ceramic particles are introduced into the Cu-based composite coatings. The metal-based ceramic composite powder has the characteristics of metal powder and the advantages of ceramics, so its physical properties are different from those of the conventional metal powder. The deposition behavior of ceramic particles also dramatically differs from that of conventional metal particles. In the CS process, the ceramic particles as a hard reinforcing phase can hardly deform, while the metallic phase is prone to deformation due to the ASI, thus forming metallurgical and mechanical bonding with the substrate
[2][17][24,39]. Hence, investigating the interfacial bonding process and deposition mechanism of the ceramic composite coatings is of vital significance to improve the performance of cold-sprayed Cu-based ceramic composite coatings.
3.2. Deposition Mechanism of Cu-Based Ceramic Composite Coatings
It has been revealed that the hardness, density, wear resistance, and mechanical properties of cold-sprayed coatings can be greatly enhanced and their bearing capacity can be strengthened through the addition of a hard reinforcing phase to the coatings
[18][40]. Hence, the researchers added relatively harder metal particles and ceramic particles into metal feedstock particles, so as to analyze the role of the reinforcing phase in the deposition mechanism of metal-based composite coatings
[19][41]. Fernandez et al.
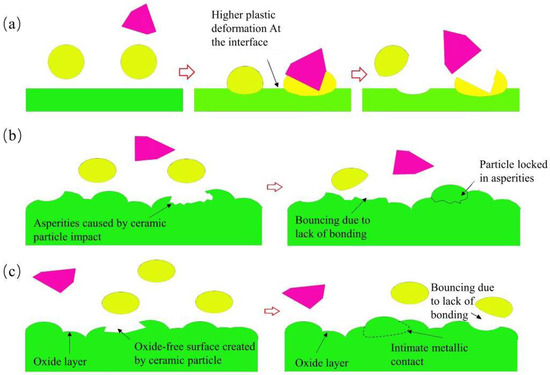
[20][42] proposed three hypotheses for the deposition mechanism of metal-based ceramic composite coatings to explain the increased deposition efficiency of coatings, including the shot peening (SP) effect, sandblasting effect, and cleaning effect. With ceramic particles as shots, the preceding soft particles are shot peened to increase their degree of deformation, thus increasing the deposition efficiency of the softer metal particles (
Figure 2a). With the increase of the ceramic content, the interface between the coatings and the substrate becomes rougher due to the sandblasting effect of the ceramic particles on the substrate and the deposited particles (
Figure 2b). When ceramic particles impact the metal or substrate, the oxide film on the surface of the metal particles or substrate is cleaned due to deformation, and the fresh metal surface can be exposed for the subsequent bonding of metal particles to produce more metallurgical bonding areas. Hence, ceramic particles can raise the deposition efficiency of metal particles (
Figure 2c).
Figure 2. Schematic diagram of the three effects of metal–ceramic particles during deposition
[20][42]. (
a) Shot blasting effect; (
b) sand blasting effect; (
c) cleaning effect.
He and Chen et al.
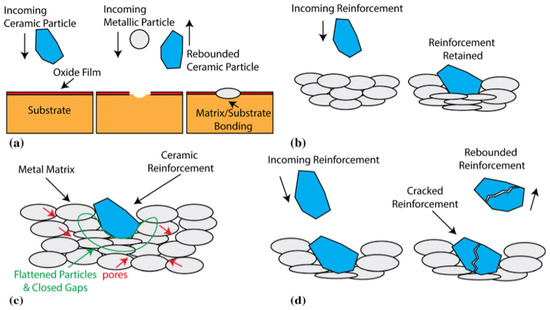
[21][22][43,44] summarized the impact effect between ceramic particles and metal particles in their research and concluded four effects according to the addition order of ceramic particles, namely, the surface activation effect, the cushioning effect, the tamping effect, and the erosion effect (
Figure 3). Firstly, the high-velocity impact of ceramic particles destroys the oxide layer on the substrate surface, and the pits generated by the impact increase the roughness of the substrate, so that the substrate surface maintains high activity (
Figure 3a). Secondly, the metal surface can effectively accommodate plastic deformation, decrease the impact kinetic energy of the ceramic particles, provide an embedding mechanism for hard ceramic particles, and exert a buffering effect on the substrate (
Figure 3b). Thirdly, with the formation of coatings, the continuous impact of the ceramic particles exerts a compression or SP effect, thus reducing the coating porosity and enhancing the metallurgical bonding between the metallic phase and the metal/ceramic interface (
Figure 3c). At last, with the elevation of the number of ceramic particles, ceramic particles are vulnerable to fragmentation by impacts, and the erosion effect produced thereof exerts an adverse effect on deposition (
Figure 3d).
Figure 3. Schematic diagram of four effects of hard ceramic particles during deposition
[21][43]. (
a) Surface activation; (
b) cushioning effect of the matrix; (
c) tamping effect of ceramic reinforcement; (
d) erosion effect.
According to the research above, the Cu-based composite coatings can be strengthened by the addition of ceramic particles during the CS from the following aspects: (1) Ceramic particles can activate metal particles by removing the oxide layer on their surface, which increases the deposition efficiency. (2) Ceramic particles can fill the gaps between metal particles and increase the compactness of composite coatings. (3) Ceramic particles play a role in compacting metal particles, which not only increases the hardness and bonding strength of the Cu-based composite coatings, but also improves the mechanical properties of the coatings. Therefore, it is of great value to make rational use of the reinforcing effect of ceramic particles to improve the mechanical properties of Cu-based composite coatings. Current research has provided no complete theoretical system elaborating the bonding mechanism of Cu-based ceramic composite coatings, mainly because the physical properties of Cu in the metallic phase and ceramic particles in the reinforcing phase are quite different. In addition, the impact time is very short (tens of nanoseconds), and the experimental technology is limited, so it is impossible to capture the specific impact and bonding process of ceramic particles. However, in recent years, researchers have been able to observe the impact and bonding process between ceramic particles in real time using in-situ scanning electron microscopy and femto-photography. The discovery of this process can provide technical support for the design, preparation, and application of high-performance Cu-based ceramic composite coatings.